Suspension
StructurePolyvinyl Chloride (PVC) suspension grade is a thermoplastic polymer produced through the suspension polymerization process. In this method, vinyl chloride monomer (VCM) is dispersed in water with the help of suspending agents and polymerized using free radical initiators. The resulting PVC resin consists of fine, porous, and free-flowing particles with a relatively high molecular weight, making it suitable for a wide range of applications. The polymer structure is primarily composed of repeating vinyl chloride units (–CH₂–CHCl–), forming a linear polymer chain with varying degrees of polymerization. PVC suspension grade is widely used in the manufacturing of pipes, fittings, films, sheets, and rigid as well as flexible products due to its excellent mechanical strength, durability, and chemical resistance. The properties of the resin, such as particle size, porosity, and bulk density, can be adjusted by controlling the polymerization conditions, making it versatile for different industrial applications.
PropertiesPVC suspension grade exhibits a combination of excellent physical, mechanical, and chemical properties, making it highly versatile for industrial applications. It appears as a white, free-flowing powder with a bulk density ranging from 0.45 to 0.65 g/cm³ and a particle size typically between 50-250 microns. Its high porosity allows for better plasticizer absorption, making it suitable for both rigid and flexible products. Mechanically, it offers good tensile strength, typically between 40-60 MPa, and moderate to high impact resistance, which can be enhanced with additives. Chemically, PVC suspension grade is highly resistant to acids, bases, and many chemicals, ensuring durability in harsh environments. It also has low water absorption, which provides excellent dimensional stability. However, it is susceptible to UV degradation, requiring stabilizers for outdoor applications. These properties make PVC suspension grade ideal for manufacturing pipes, profiles, films, and various other rigid and flexible products.
Applications
- Construction Industry: Pipes, fittings, window profiles, doors, roofing sheets
- Packaging Industry: Films, sheets, bottles
- Automotive Industry: Interior trims, dashboards, wire insulation
- Medical Sector: Tubing, blood bags, IV containers
- Electrical Applications: Cable insulation, coatings
Advantages
- High durability and strength – Ideal for long-term use
- Excellent chemical resistance – Withstands acids, bases, and oils
- Cost-effective – Affordable compared to other polymers
- Low water absorption – Ensures dimensional stability
- Easily processable – Can be molded, extruded, and shaped easily
- Customizable – Properties can be modified with additives
Disadvantages
- UV degradation – Becomes brittle under prolonged sunlight exposure
- Toxic gas release – Emits harmful gases (HCl) when burned
- Health concerns – Some plasticizers used in flexible PVC may have risks
- Not biodegradable – Raises environmental concerns regarding disposal
Synthetic Rubber Latex
Properties of synthetic latexFlexibility: Synthetic latex becomes a flexible film after drying. Adhesion: Synthetic latex adheres to many surfaces and forms a seamless layer. Abrasion resistance: Some types of synthetic latex have high abrasion resistance. Heat and sound insulation: Synthetic latex is a good heat and sound insulation. Resistance to chemicals: Some types of synthetic latex are resistant to chemicals.
Applications of synthetic latexRubber industry: Production of car tires, shoe soles, belts and other rubber products Textile industry: Production of coated fabrics and textile adhesives Construction industry: Production of latex paints, tile and ceramic adhesives, thermal and acoustic insulation Medical industry: Production of surgical gloves, bandages and other medical products Paper industry: Production of coated papers and paper adhesives
Tackifiers
Why do we need adhesives?Increasing initial adhesion: Tackifiers cause materials to stick together quickly and prevent them from slipping. Improving adhesion properties: These substances can improve other properties of the adhesive, such as flexibility, heat resistance, and chemical resistance. Adjusting viscosity: Tackifiers can adjust the viscosity (thickness) of the adhesive, allowing it to spread easily on different surfaces.
Types of AdhesivesAdhesives are divided into different types based on their origin and chemical structure: Natural adhesives: such as natural resins (such as pine resin), vegetable oils and waxes Synthetic adhesives: such as petrochemical resins (such as coumarone-indene resins), esters and synthetic polymers. Modified adhesives: Natural or synthetic adhesives that have been modified to improve their properties. Mechanism of action of adhesives Adhesives increase adhesion by creating intermolecular forces between material particles. These forces can be van der Waals forces, hydrogen bonds or chemical bonds. Van der Waals forces: These forces exist between all molecules and are the weakest type of intermolecular forces. Hydrogen bonds: These bonds are created between molecules that have a hydrogen atom attached to an electronegative atom (such as oxygen or nitrogen). Chemical bonds: These bonds are the strongest type of bonds and are created in some adhesives.
Applications of adhesivesAdhesive industry: In the production of pressure-sensitive adhesives, construction adhesives, industrial adhesives, etc. Ink industry: In the production of printing inks, industrial inks, etc. Asphalt industry: In the production of asphalt to improve adhesion between aggregates and bitumen. Rubber industry: In the production of adhesive rubbers and sealants.
Advantages of using adhesivesIncrease the speed of adhesion: Causes objects to adhere to each other quickly. Increase the strength of adhesion: Causes the adhesion created to be stronger and more stable. Improving the physical properties of the adhesive: Improves the flexibility, heat resistance, and chemical resistance of the adhesive.
Tap Stabilizer
tartaric acid
Tartaric acid is a naturally occurring organic compound with the chemical formula C₄H₆O₆, widely found in fruits such as grapes and tamarind. It appears as white, odorless, water-soluble crystals and is considered one of the most important organic acids in the food, pharmaceutical, and chemical industries.
In industry, it is especially used as a pH regulator, antioxidant, and stabilizer, and it is present in a variety of products, from beverages to effervescent medications.
Structure of Tartaric Acid
Tartaric acid contains two carboxylic acid groups (–COOH) and two hydroxyl groups (–OH). Its structure includes two chiral centers, meaning it can exist in different isomeric forms, including L(+), D(−), and DL (racemic) forms. The naturally occurring and biologically active form is L(+)-tartaric acid.
Properties of Tartaric Acid
-
Molecular Formula: C₄H₆O₆
-
Molar Mass: 150.09 g/mol
-
Appearance: White powder or crystals
-
Water Solubility: Soluble
-
Taste: Sour
-
Melting Point: ~170 °C
-
Metal Complexing Ability: High (forms complexes with metal ions such as potassium and calcium)
Applications of Tartaric Acid
✅ Food Industry
✅ Pharmaceutical Industry
✅ Chemical and Metal Industry
✅ Cosmetic Industry
Disadvantages of Tartaric Acid
⚠️ In high doses, it may cause gastrointestinal irritation
⚠️ Potential for allergic reactions in sensitive individuals
⚠️ Decomposes at high temperatures and may lose functionality in thermal processes
⚠️ Inhalation of the powder may irritate the respiratory tract
Advantages of Tartaric Acid
✅ Natural and biodegradable
✅ Compatible with food and pharmaceutical products
✅ Enables precise pH adjustment
✅ High stability in stored products
✅ Extends shelf life of food products
TetrafluoroEthylene/perfluoroPropylene copolymers (FEP)
StructureThe structure of Tetrafluoroethylene/Perfluoropropylene (FEP) copolymer consists of a randomly distributed backbone of tetrafluoroethylene (TFE) and hexafluoropropylene (HFP) monomer units. The TFE units provide the high thermal and chemical resistance characteristic of fluoropolymers, while the HFP units introduce branching that disrupts crystallinity, enhancing flexibility and melt processability. The polymer chain is composed of repeating –CF₂–CF₂– segments from TFE and –CF₂–CF(CF₃)– segments from HFP, where the bulky trifluoromethyl (-CF₃) groups reduce intermolecular forces, lowering the melting point compared to PTFE. This molecular architecture results in a copolymer with excellent non-stick properties, chemical inertness, and transparency while being more easily processed using conventional melt-processing techniques.
PropertiesTetrafluoroethylene/Perfluoropropylene (FEP) copolymers exhibit a unique combination of thermal stability, chemical resistance, electrical insulation, and mechanical flexibility. They can withstand continuous exposure to high temperatures up to 200°C (392°F) while maintaining their structural integrity. FEP is highly resistant to a wide range of chemicals, including acids, bases, and organic solvents, making it ideal for harsh environments. Its non-stick and low-friction surface prevents adhesion and contamination, similar to PTFE. Unlike PTFE, FEP is melt-processable, allowing for fabrication through extrusion, injection molding, and blow molding. It also possesses excellent electrical insulating properties, with a low dielectric constant and high breakdown voltage, making it a preferred choice for wire and cable insulation. Additionally, FEP is optically transparent, resistant to UV radiation, and does not degrade under prolonged exposure to environmental factors, further enhancing its suitability for industrial, aerospace, and medical applications.
Applications of FEP Copolymers:
- Wire & Cable Insulation: Used in aerospace, automotive, and telecommunications due to high heat and chemical resistance.
- Chemical Processing Equipment: Linings for pipes, valves, and tanks in harsh chemical environments.
- Medical Tubing & Catheters: Biocompatible and resistant to sterilization processes.
- Food & Beverage Industry: Non-stick coatings for cooking equipment and food processing machinery.
- Semiconductor Industry: Used in chip manufacturing equipment due to high purity and chemical resistance.
- Heat Shrink Tubing: Provides electrical insulation and protection in extreme environments.
- Optical Fiber Coatings: Protects fibers in harsh conditions without affecting signal transmission.
- Laboratory Equipment: Used for beakers, flasks, and other chemical-resistant lab tools.
Advantages of FEP Copolymers:
- Excellent Chemical Resistance: Inert to most acids, bases, and solvents.
- High Thermal Stability: Can withstand temperatures up to ~200°C (392°F).
- Non-Stick Properties: Similar to PTFE (Teflon), preventing adhesion of substances.
- Low Friction: Reduces wear in moving parts and improves efficiency.
- Electrical Insulation: High dielectric strength makes it ideal for electrical applications.
- Transparent & UV Resistant: Can be used in optical and outdoor applications.
- Biocompatibility: Safe for medical and food-contact applications.
Disadvantages of FEP Copolymers:
- Lower Mechanical Strength: Weaker than PTFE in terms of tensile strength and wear resistance.
- Higher Cost: More expensive than common plastics like PVC or polyethylene.
- Limited Temperature Resistance: Slightly lower thermal stability than PTFE.
- Difficult Processing: Requires specialized molding and extrusion techniques.
- Fluorine Emission on Decomposition: Can release toxic fumes if overheated beyond its thermal limits.
Tetrapotassium pyrophosphate
Tetrasodium pyrophosphate
Textile Grade
- Molecular Structure:
- Natural fibers have a cellulose-based (plant fibers) or protein-based (animal fibers) molecular structure.
- Synthetic fibers are often derived from polymer chains, such as polyethylene terephthalate (PET) in polyester.
- Fiber Morphology:
- Fibers are classified as filament (long, continuous fibers like silk) or staple (short fibers like cotton).
- Processing techniques such as spinning, weaving, and knitting alter fiber orientation for enhanced strength and flexibility.
- Surface Treatment:
- Textile grade materials undergo chemical treatments such as dyeing, anti-static coating, moisture-wicking finishes, and UV protection to enhance their functionality.
PropertiesTextile grade materials possess distinct characteristics that make them suitable for various applications:
1. Mechanical Properties
✔ High Tensile Strength – Ensures durability and resistance to tearing. ✔ Elasticity – Some fibers (like spandex) stretch and recover their shape. ✔ Abrasion Resistance – Important for heavy-use applications like upholstery and workwear.2. Thermal Properties
✔ Heat Resistance – Some fibers (e.g., aramid, wool) withstand high temperatures. ✔ Low Melting Point – Certain synthetics (e.g., polyester) require controlled heat processing.3. Chemical Properties
✔ Moisture-Wicking – Polyester and nylon repel moisture, keeping fabrics dry. ✔ Chemical Resistance – Some textile grades resist acids, alkalis, and solvents. ✔ UV Protection – Certain fibers (e.g., acrylic) naturally block UV rays.4. Environmental Properties
✔ Biodegradability – Natural fibers decompose over time, unlike synthetics. ✔ Sustainability – Eco-friendly textile grades include organic cotton, recycled polyester, and bamboo fibers.ApplicationsTextile grade materials are used across multiple industries, including fashion, home textiles, and technical applications.
1. Apparel Industry
- Clothing – Shirts, pants, jackets, and sportswear.
- Performance Fabrics – Moisture-wicking athletic wear, compression garments.
- Luxury Textiles – Silk, high-thread-count cotton, and premium synthetic blends.
2. Home Textiles
- Upholstery – Durable fabrics for sofas, curtains, and carpets.
- Bedding – Sheets, blankets, and pillowcases.
- Towels & Linens – Cotton-based materials for comfort and absorbency.
3. Industrial & Technical Textiles
- Automotive Textiles – Car seats, airbags, and interior linings.
- Medical Textiles – Surgical gowns, bandages, and antimicrobial fabrics.
- Protective Gear – Fire-resistant clothing (e.g., Nomex), bulletproof vests (Kevlar).
4. Eco-Friendly & Sustainable Applications
- Recycled Textiles – Upcycled polyester from plastic bottles.
- Organic Fibers – Bamboo, hemp, and organic cotton for sustainable fashion.
AdvantagesDurability – Textile grade materials are engineered for longevity. Versatility – Suitable for various applications, from fashion to industry. Lightweight & Comfortable – Many textiles are breathable and easy to wear. Easy Maintenance – Many fibers resist stains, wrinkles, and shrinkage. Cost-Effective – Mass production of synthetics reduces costs. Sustainability Options – Growing availability of eco-friendly textile grades.
DisadvantagesEnvironmental Concerns – Many synthetic fibers are non-biodegradable and contribute to plastic pollution. Flammability – Some textiles require fire-resistant treatments for safety. Chemical Sensitivity – Certain materials degrade when exposed to strong detergents, acids, or UV light. Heat Sensitivity – Synthetic fibers like polyester melt at high temperatures, limiting ironing options. Moisture Absorption Issues – Some fibers retain or repel moisture excessively, impacting comfort.
Thermoplastic Copolyesters (COPE)/(TPEE)
PropertiesThermoplastic Copolyesters (COPE), also known as Thermoplastic Polyester Elastomers (TPEE), combine the mechanical strength of engineering plastics with the flexibility and resilience of elastomers. They exhibit excellent elasticity, allowing them to return to their original shape after deformation, while also providing high tensile strength and durability. COPE materials offer outstanding chemical and solvent resistance, making them suitable for harsh environments. Their thermal stability enables them to maintain performance across a wide temperature range, with good low-temperature flexibility and resistance to heat aging. Additionally, they possess excellent abrasion resistance, impact strength, and fatigue resistance, ensuring longevity in demanding applications. With easy processability through injection molding, extrusion, and blow molding, COPE is widely used in automotive, industrial, consumer, and medical applications where a balance of toughness, flexibility, and chemical resistance is required.
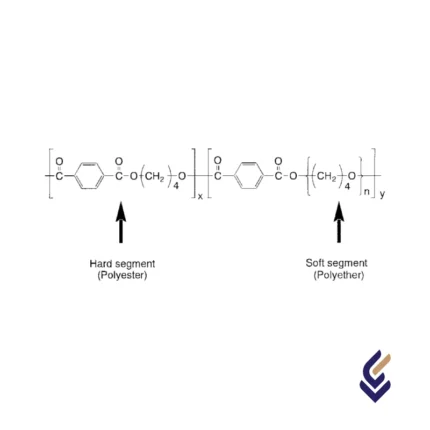
StructureThermoplastic Copolyesters (COPE), also known as Thermoplastic Polyester Elastomers (TPEE), are a class of high-performance elastomers that combine the characteristics of both thermoplastics and rubbers. Their structure consists of alternating soft and hard segments, where the soft segments are typically made of aliphatic polyether or polyester, providing flexibility and elasticity, while the hard segments are composed of polyester blocks, offering strength, thermal resistance, and durability. This segmented block copolymer structure enables TPEEs to exhibit excellent mechanical properties, such as high tensile strength, impact resistance, and superior fatigue endurance. The presence of ester linkages in the hard phase contributes to chemical resistance and heat stability, while the soft phase ensures flexibility even at low temperatures. Due to this unique molecular architecture, COPEs find applications in various industries, including automotive, consumer goods, electrical components, and medical devices, where both resilience and processability are essential.
Applications
- Automotive: Used in air ducts, CVJ boots, bellows, gaskets, and wire coatings due to high heat and chemical resistance.
- Industrial & Mechanical: Employed in conveyor belts, hoses, seals, and grommets for durability and flexibility.
- Consumer Goods: Found in footwear soles, sports equipment, and flexible smartphone components for comfort and toughness.
- Electrical & Electronics: Used in cable insulation, connectors, and protective coatings due to excellent dielectric properties.
- Medical Devices: Applied in tubing, catheters, and soft-touch grips because of biocompatibility and sterilization resistance.
Advantages
- High Elasticity & Flexibility: Maintains shape and flexibility even under stress.
- Excellent Heat Resistance: Performs well at elevated temperatures compared to other TPEs.
- Superior Mechanical Strength: Offers high tensile strength, impact resistance, and fatigue endurance.
- Good Chemical Resistance: Resistant to oils, solvents, and many industrial chemicals.
- Wide Processing Window: Easily processed through injection molding, extrusion, and blow molding.
- Recyclable: More environmentally friendly than thermoset elastomers.
Disadvantages
- Higher Cost: More expensive than other thermoplastic elastomers (TPEs).
- Limited Low-Temperature Flexibility: Can become less flexible at extremely low temperatures compared to TPU.
- Absorbs Moisture: May require drying before processing to prevent defects.
- Processing Challenges: Requires precise temperature control during molding and extrusion
ThermoPlastic Elastomer
Applications of TPEsTPEs are used in a variety of industries due to their unique properties, including: Automotive industry: Interior parts of cars such as gear levers, seat covers, and under-hood parts. Medical industry: Medical gloves, medical tubing, and other medical equipment. Sports industry: Athletic shoes, balls, and other sports equipment. Packaging: Flexible packaging, airbags, and protective packaging. Home use: Home appliances, toys, and other consumer products. Electronic industry: Protective coverings for cables and electronic components.
ThermoPlastic Elastomer PVC (TPVC)
Structure and Properties of TPVCTPVCs are usually block copolymers consisting of soft blocks (such as polyether) and hard blocks (such as PVC). This unique structure gives TPVC the following properties: Very high chemical resistance: It exhibits excellent resistance to a wide range of chemicals, including acids, bases, solvents, and oils. Heat resistance: It maintains its stability at high temperatures and is resistant to oxidation. Weather resistance: It is resistant to ozone and ultraviolet radiation, and as a result, it has a longer life. Resistance to oils and solvents: It shows very good resistance to oils and organic solvents. Good electrical properties: It has good insulating properties and is used in the electronics industry. Moldability: TPVC can be easily formed using conventional thermoplastic molding methods.
TPVC applicationsTPVC is used in specific industries and special applications due to its unique properties, including: Automotive industry: Production of chemical-resistant gaskets, fuel pipes and engine parts. Aerospace industry: Production of parts exposed to high temperatures and corrosive environments. Chemical industry: Production of coatings and pipes that come into contact with corrosive chemicals. Electronics industry: Production of insulators and parts exposed to moisture and chemicals. Medical industry: Production of pipes and parts that come into contact with chemicals and biological materials.
Advantages of using TPVCVery high chemical resistance: Suitable for applications that require high chemical resistance. Heat resistance: It performs well in high temperature environments. Good electrical properties: Suitable for electronic applications. Excellent moldability: Mass production of products at low cost Long life: TPVC is resistant to environmental factors and has a longer life.