Nonionic Surfactant
Characteristics of Nonionic SurfactantsFoaming: Many nonionic surfactants have good foaming properties and are used in the production of shampoos, dishwashing liquid, and other cleaning products. Emulsifying: These substances can form stable emulsions between oil and water. Moisturizing: Nonionic surfactants can retain moisture in the skin and hair. Physiologically Inert: Many nonionic surfactants are gentle on the skin and hair and do not cause irritation. Compatibility with other materials: Nonionic surfactants are easily combined with other chemicals.
Types of nonionic surfactantsEthylene oxide alkylphenol: These types of surfactants are produced by the reaction of ethylene oxide with alkylphenol. Ethoxylated alcohols: These materials are obtained from the ethoxylation of fatty alcohols. Alkyl polyglucosides: These materials are derived from sugar and alcohol and are biodegradable.
Applications of nonionic surfactantsCosmetic and health industries: In the production of shampoos, dishwashing liquid, lotions, creams and other cosmetic and health products Textile industry: As a softener, moisturizer and emulsifier in the textile industry Oil industry: As an emulsifying agent in oil well drilling Agricultural industry: As an emulsifier in the formulation of agricultural pesticides Food industry: As an emulsifier in the production of some food products
Nonyl Phenol Soap mol 6 and 10
6 and 10 mole soaps6 and 10 mole soaps are actually nonylphenol ethoxylate with 6 and 10 moles of ethylene oxide. These substances were used as surfactants in many detergent products due to their emulsifying, foaming, and good water solubility properties.
Applications of 6 and 10 mole soapsDetergent industries: These materials were used as the basis of many industrial and household detergents such as dishwashing liquid, shampoo, laundry detergent and industrial cleaners. Textile industries: They were used in textile processes for softening, wetting and increasing dye absorption. Paper and pulp industries: They were used in various stages of paper production to improve quality and reduce costs. Agriculture: They were used as emulsifiers in the formulation of pesticides and fertilizers.
Why has the use of 6 and 10 mole soaps been limited?Despite their wide applications, the use of 6 and 10 mole soaps has been limited due to environmental and health concerns. Some of the reasons for this limitation include: Environmental toxicity: Nonylphenols and their derivatives are highly toxic to aquatic organisms and can cause serious damage to aquatic ecosystems. Environmental Persistence: These substances degrade slowly and can persist in the environment for long periods of time. Hormonal Disruption: Nonylphenols can disrupt the hormonal system of humans and animals, causing health problems including reproductive disorders and abnormal growth.
Nonylphenol 10 mol Ethoxylated
Structure and PropertiesStructure: Nonylphenol Ethoxylate has a hydrophobic part (nonylphenol hydrocarbon chain) and a hydrophilic part (ethylene oxide chain). This dual structure allows this material to interact with both oily substances and water. Properties: NPE-10 has emulsifying, foaming, wetting and good solubility in water. These properties have led to its use as a surfactant in many products.
Nonylphenol ethoxylate 10 mol applicationsDetergent industries: Used as the base of many industrial and household detergents such as dishwashing liquid, shampoo, laundry detergent and industrial cleaners. Textile industries: Used in textile processes for softening, moisturizing and increasing dye absorption. Paper and pulp industries: Used in various stages of paper production to improve quality and reduce costs. Agriculture: Used as an emulsifier in the formulation of agricultural pesticides and fertilizers. Paint and coating industries: Used as an emulsifier and dispersing agent in the production of paints and coatings.
Nonylphenol 10 Moles
Structure and PropertiesStructure: Nonylphenol ethoxylate has a hydrophobic part (nonylphenol hydrocarbon chain) and a hydrophilic part (ethylene oxide chain). This dual structure allows this material to interact with both oily substances and water. Properties: NPE-10 has emulsifying, foaming, wetting and good solubility in water. These properties have led to this material being used as a surfactant in many products.
Nonylphenol ethoxylate 10 mol applicationsDetergent industries: Used as the base of many industrial and household detergents such as dishwashing liquid, shampoo, laundry detergent and industrial cleaners. Textile industries: Used in textile processes for softening, moisturizing and increasing dye absorption. Paper and pulp industries: Used in various stages of paper production to improve quality and reduce costs. Agriculture: Used as an emulsifier in the formulation of agricultural pesticides and fertilizers. Paint and coating industries: Used as an emulsifier and dispersing agent in the production of paints and coatings.
Nonylphenol 6 Moles
Structure and PropertiesStructure: Nonylphenol ethoxylate has a hydrophobic part (nonylphenol hydrocarbon chain) and a hydrophilic part (ethylene oxide chain). This dual structure allows this substance to interact with both oily substances and water. Properties: NPE-6 has emulsifying, foaming, wetting and good solubility in water. These properties have led to this substance being used as a surface active agent in many products.
Nonylphenol ethoxylate 6 mol applicationsDetergent industries: Used as the base of many industrial and household detergents such as dishwashing liquid, shampoo, laundry detergent and industrial cleaners. Textile industries: Used in textile processes for softening, wetting and increasing dye absorption. Paper and pulp industries: Used in various stages of paper production to improve quality and reduce costs. Agriculture: Used as an emulsifier in the formulation of agricultural pesticides and fertilizers. Paint and coating industries: Used as an emulsifier and dispersing agent in the production of paints and coatings.
Nylon 6
PropertiesNylon 6 is a strong, lightweight, and durable engineering thermoplastic known for its excellent mechanical and thermal properties. It has high tensile strength, toughness, and impact resistance, making it suitable for demanding applications. It also exhibits good wear resistance, low friction, and excellent abrasion resistance, which enhances its longevity in mechanical parts. Nylon 6 has a melting point of approximately 220°C and maintains stability over a wide temperature range. It offers good chemical resistance to oils, greases, and many solvents but is sensitive to strong acids and bases. One of its notable characteristics is its high moisture absorption, which can affect its mechanical strength and dimensional stability. Nylon 6 also has good electrical insulating properties, making it useful in electrical and electronic applications. Additionally, it is easily processable through injection molding, extrusion, and fiber spinning, allowing for its widespread use in textiles, automotive components, and industrial applications.
StructureNylon 6 is a synthetic polymer belonging to the polyamide family, characterized by its repeating units derived from caprolactam through a ring-opening polymerization process. The molecular structure of Nylon 6 consists of a linear chain of amide (–CONH–) linkages interspersed with six-carbon alkyl segments, forming a highly regular and symmetrical backbone that contributes to its notable mechanical strength, thermal stability, and chemical resistance. Unlike Nylon 6,6, which is synthesized from two different monomers, Nylon 6 is synthesized from a single monomer, ε-caprolactam, which undergoes polymerization through successive opening of the lactam ring, resulting in a continuous chain structure. The presence of hydrogen bonding between adjacent polymer chains enhances intermolecular interactions, leading to high crystallinity and improved tensile properties. This structural arrangement imparts Nylon 6 with desirable characteristics such as high flexibility, durability, and resistance to abrasion, making it widely utilized in textiles, engineering plastics, and industrial applications.
Applications of Nylon 6
- Textiles and Fabrics: Nylon 6 is widely used in the textile industry to produce items such as hosiery, swimwear, activewear, and undergarments due to its elasticity, strength, and smooth texture.
- Industrial Uses: Its high tensile strength and abrasion resistance make Nylon 6 suitable for manufacturing ropes, fishing nets, conveyor belts, and tire cords.
- Automotive Components: Nylon 6 is utilized in producing various automotive parts, including gears, bearings, and under-the-hood components, because of its durability and thermal stability.
- Consumer Goods: Common household items like toothbrush bristles, combs, and kitchen utensils are often made from Nylon 6 due to its resilience and ease of molding.
- Engineering Plastics: Nylon 6 is used in the production of engineering plastics for applications such as gears, bearings, and other mechanical components, owing to its strength and wear resistance.
Advantages of Nylon 6
- High Strength and Durability: Nylon 6 exhibits excellent tensile strength, making it suitable for products requiring long-lasting performance.
- Flexibility and Elasticity: The material offers good flexibility and can return to its original shape after stretching, which is beneficial for textile applications.
- Chemical Resistance: Nylon 6 is resistant to a wide range of chemicals, including oils and solvents, enhancing its suitability for various industrial applications.
- Thermal Resistance: With a high melting point, Nylon 6 can withstand elevated temperatures, making it appropriate for applications involving heat exposure.
- Lightweight: Nylon 6 is lighter than many metals, which is advantageous in applications where weight reduction is desired.
Disadvantages of Nylon 6
- Moisture Absorption: Nylon 6 is hygroscopic and can absorb moisture from the environment, leading to dimensional changes and potential degradation of mechanical properties.
- UV Sensitivity: Prolonged exposure to ultraviolet light can cause Nylon 6 to degrade, leading to discoloration and loss of strength.
- Lower Impact Resistance: Compared to some other engineering plastics, Nylon 6 may exhibit lower impact resistance, which could limit its use in high-impact applications.
- Processing Challenges: Nylon 6 requires careful control during processing, as it is sensitive to moisture and can degrade if not properly dried before molding.
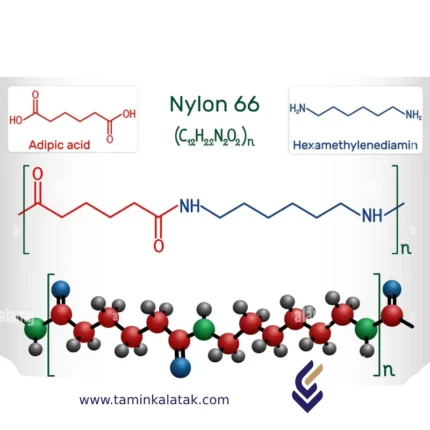
Nylon 6-6
StructureNylon 66 is a synthetic polyamide with a repeating molecular structure formed through the condensation polymerization of hexamethylenediamine and adipic acid. The polymer consists of amide (-CONH-) linkages connecting alternating units of six carbon atoms from each monomer, resulting in a linear, highly ordered structure. This arrangement allows strong hydrogen bonding between polymer chains, enhancing its strength, rigidity, and thermal resistance. The repeating unit in Nylon 66 contains both aliphatic and amide groups, contributing to its balanced combination of flexibility and toughness. The presence of these intermolecular forces gives Nylon 66 its high melting point, excellent wear resistance, and mechanical stability, making it a widely used material in engineering and industrial applications.
PropertiesNylon 66 exhibits a combination of excellent mechanical, thermal, and chemical properties, making it highly suitable for various industrial applications. It has high tensile strength, toughness, and rigidity, which contribute to its durability and resistance to wear and abrasion. Its high melting point, typically around 255°C, allows it to maintain structural integrity under elevated temperatures. Nylon 66 also has good chemical resistance, particularly against oils, solvents, and many hydrocarbons, though it can absorb moisture, which may affect its mechanical properties. It has low friction and self-lubricating properties, making it ideal for applications requiring smooth movement and reduced wear. Additionally, Nylon 66 has good electrical insulation properties, making it useful in electrical and electronic components. Its ability to be easily molded and processed further enhances its versatility in manufacturing.
Applications of Nylon 66
- Automotive parts such as gears, bearings, fuel lines, and radiator tanks
- Electrical and electronic components like connectors, cable ties, and insulators
- Industrial machinery components, including conveyor belts and mechanical fasteners
- Textiles and fibers used in carpets, ropes, parachutes, and outdoor wear
- Consumer goods such as sports equipment, kitchen utensils, and zippers
- Packaging materials, especially in films and coatings for food and medical applications
Advantages of Nylon 66
- High tensile strength and durability
- Excellent resistance to wear, abrasion, and impact
- High melting point and good thermal stability
- Good chemical resistance to oils, solvents, and hydrocarbons
- Low friction and self-lubricating properties
- Good electrical insulation properties
- Easily moldable and processable for various applications
Disadvantages of Nylon 66
- Absorbs moisture, which can affect mechanical and dimensional stability
- Can degrade under prolonged exposure to UV light without proper additives
- More expensive than other types of nylon, such as Nylon 6
- Can be attacked by strong acids and bases
- High processing temperature required for manufacturing
Oleic Acid
Oleic Acid is an unsaturated omega-9 fatty acid naturally found in many animal and vegetable fats and oils. It is widely used in the chemical, cosmetic, pharmaceutical, and food industries. Its chemical formula is C₁₈H₃₄O₂, and at room temperature, it appears as a colorless to pale yellow oily liquid.
Chemical Structure of Oleic Acid
Oleic acid consists of 18 carbon atoms with a single double bond located at the ninth carbon (cis-9-octadecenoic acid). This structure results in specific physical properties such as a low melting point and fluidity at room temperature.
-
Molecular Formula: C₁₈H₃₄O₂
-
Molecular Weight: 282.47 g/mol
-
Functional Group: Carboxylic acid
-
Double Bond Position: C9=C10 (cis configuration)
Properties of Oleic Acid
-
Colorless to pale yellow liquid at room temperature
-
Soluble in alcohol, ether, and most organic solvents
-
Non-volatile, odorless or slightly fatty odor
-
Melting Point: 13–14°C
-
Boiling Point: 360°C (decomposes)
-
Exhibits emulsifying and natural lubricating properties
Applications of Oleic Acid
Due to its unique properties, oleic acid is used across various industries:
✅ Cosmetic and Personal Care Industry: Used in creams, lotions, soaps, and emulsions
✅ Food Industry: As a permitted fatty additive and flavoring agent
✅ Pharmaceutical Industry: As a drug carrier or lipophilic solvent
✅ Chemical Industry: As a raw material for surfactants and esters
✅ Industrial Lubricants: In the formulation of metal and industrial lubricants
✅ Plastic and Rubber Manufacturing: Used as a plasticizer
Disadvantages of Oleic Acid
🔻 May cause skin irritation in some individuals when applied topically
🔻 Limited thermal stability at high temperatures
🔻 Excessive dietary intake may contribute to weight gain
🔻 Can solidify or precipitate at low temperatures
Advantages of Oleic Acid
✅ Biodegradable and environmentally friendly
✅ Non-toxic and safe for pharmaceutical and cosmetic applications
✅ Widely available and cost-effective
✅ Strong emulsifying and softening properties
✅ Can be blended with other chemical compounds for advanced product formulations
On-line & Semi-finished Products T…
Open Mill
Organic compound
Other Compounding Agents for Latex
Key Compounding Agents for LatexStabilizers: These are added to increase the electrostatic stability of the latex. Common stabilizers include pH builders like ammonia and potassium hydroxide (KOH), as well as surfactants such as potassium oleate and ammonium laureate. Vulcanizing Agents: These chemicals form crosslinks between rubber chains, enhancing the elasticity and strength of the latex. The primary vulcanizing agent is sulfur, but other agents like zinc oxide (ZnO) and magnesium oxide (MgO) are used for specific types of rubber1. Vulcanizing Accelerators: These compounds increase the rate of vulcanization, allowing the process to occur at lower temperatures and with greater efficiency. Examples include tetramethyl thiuram disulfide (TMTD) and organic peroxides1. Activators: These are used to enhance the effectiveness of vulcanizing agents and accelerators. Antioxidants: These additives prevent the degradation of latex by inhibiting the oxidation process, thereby extending the shelf life and durability of the product. Fillers: These are added to improve the mechanical properties and reduce the cost of the latex. Common fillers include carbon black and calcium carbonate1. Pigments: These provide color to the latex, making it suitable for various aesthetic applications. Surfactants: These are used to improve the dispersion of other additives in the latex and enhance its overall properties.
Applications of Compounded LatexTires and Automotive Parts: Enhanced durability and performance. Medical Gloves and Prosthetics: Improved elasticity and strength. Textiles and Coatings: Superior flexibility and resistance to wear. Household Products: Long-lasting and aesthetically pleasing materials.
Benefits of Using Compounding AgentsEnhanced Performance: Improved mechanical properties, elasticity, and durability. Cost-Effective: Reduces the overall cost by optimizing the use of raw materials. Versatility: Suitable for a wide range of applications, from industrial to consumer products. Environmental Impact: Some agents are designed to be environmentally friendly, reducing the ecological footprint.