Trisodium phosphate
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Vestibulum sagittis orci ac odio dictum tincidunt. Donec ut metus leo. Class aptent taciti sociosqu ad litora torquent per conubia nostra, per inceptos himenaeos. Sed luctus, dui eu sagittis sodales, nulla nibh sagittis augue, vel porttitor diam enim non metus. Vestibulum aliquam augue neque. Phasellus tincidunt odio eget ullamcorper efficitur. Cras placerat ut turpis pellentesque vulputate. Nam sed consequat tortor. Curabitur finibus sapien dolor. Ut eleifend tellus nec erat pulvinar dignissim. Nam non arcu purus. Vivamus et massa massa.
Truck & Bus Tires
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Vestibulum sagittis orci ac odio dictum tincidunt. Donec ut metus leo. Class aptent taciti sociosqu ad litora torquent per conubia nostra, per inceptos himenaeos. Sed luctus, dui eu sagittis sodales, nulla nibh sagittis augue, vel porttitor diam enim non metus. Vestibulum aliquam augue neque. Phasellus tincidunt odio eget ullamcorper efficitur. Cras placerat ut turpis pellentesque vulputate. Nam sed consequat tortor. Curabitur finibus sapien dolor. Ut eleifend tellus nec erat pulvinar dignissim. Nam non arcu purus. Vivamus et massa massa.
Urea 46% (Prilled & Granular)
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Vestibulum sagittis orci ac odio dictum tincidunt. Donec ut metus leo. Class aptent taciti sociosqu ad litora torquent per conubia nostra, per inceptos himenaeos. Sed luctus, dui eu sagittis sodales, nulla nibh sagittis augue, vel porttitor diam enim non metus. Vestibulum aliquam augue neque. Phasellus tincidunt odio eget ullamcorper efficitur. Cras placerat ut turpis pellentesque vulputate. Nam sed consequat tortor. Curabitur finibus sapien dolor. Ut eleifend tellus nec erat pulvinar dignissim. Nam non arcu purus. Vivamus et massa massa.
Urea/Formaldehyde resins (UF)
Urea-formaldehyde (UF) resins are a class of thermosetting polymers formed by the reaction of urea and formaldehyde. They are widely used as adhesives, molding compounds, and finishes due to their high strength, hardness, and cost-effectiveness.
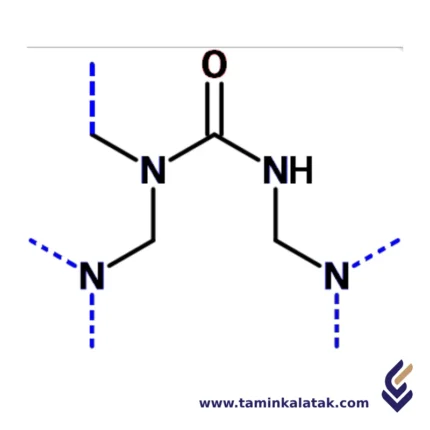
Structure Urea/Formaldehyde resins
Urea-formaldehyde (UF) resins have a complex three-dimensional network structure formed through the polymerization of urea and formaldehyde. Initially, formaldehyde reacts with urea in a stepwise condensation reaction, forming hydroxymethylated urea derivatives. These intermediates further undergo polycondensation, creating methylene (-CH2-) and methylene ether (-CH2OCH2-) linkages that interconnect the molecules. As the reaction progresses, cross-linking increases, resulting in a rigid, highly branched, and thermosetting polymer. The final cured resin consists of an extensive network of interconnected urea and formaldehyde units, giving it high strength and durability. However, the presence of residual unreacted formaldehyde can lead to emissions, which is a concern in certain applications.Properties Urea/Formaldehyde resins
Urea-formaldehyde resins possess a range of properties that make them suitable for various applications. They exhibit high tensile strength, hardness, and rigidity, making them ideal for use in adhesives and molded products. These resins have good thermal resistance but are sensitive to prolonged exposure to moisture, which can lead to degradation over time. They are lightweight and provide excellent surface finish, contributing to their widespread use in wood-based panel products such as plywood and medium-density fiberboard. Urea-formaldehyde resins cure quickly and are cost-effective, but they can be brittle and prone to cracking under stress. One of their major drawbacks is the emission of formaldehyde gas, which raises environmental and health concerns. To address this, modified formulations and formaldehyde scavengers are often used to reduce emissions while maintaining their desirable mechanical and adhesive properties.Applications of Urea-Formaldehyde Resins
- Used as adhesives in plywood, particleboard, and medium-density fiberboard (MDF) manufacturing.
- Employed in molding compounds for electrical fittings, buttons, and household items.
- Used as a surface coating in textiles, paper, and laminates to enhance durability.
- Found in insulation materials, including certain types of foams for thermal insulation.
- Utilized in the automotive and construction industries for bonding and finishing applications.
Advantages of Urea-Formaldehyde Resins
- High strength and rigidity, making them suitable for load-bearing applications.
- Fast curing properties, improving manufacturing efficiency.
- Cost-effective compared to other synthetic resins like phenol-formaldehyde.
- Excellent adhesion to wood and other porous materials.
- Good electrical insulation properties, making them useful in electrical components.
Disadvantages of Urea-Formaldehyde Resins
- Brittle nature, leading to cracks under mechanical stress.
- Poor moisture resistance, causing degradation in humid conditions.
- Formaldehyde emissions, which raise health and environmental concerns.
- Limited flexibility, making them unsuitable for applications requiring elasticity.
- Reduced durability compared to phenolic resins, especially under prolonged heat or moisture exposure.
V-belt Machine
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Vestibulum sagittis orci ac odio dictum tincidunt. Donec ut metus leo. Class aptent taciti sociosqu ad litora torquent per conubia nostra, per inceptos himenaeos. Sed luctus, dui eu sagittis sodales, nulla nibh sagittis augue, vel porttitor diam enim non metus. Vestibulum aliquam augue neque. Phasellus tincidunt odio eget ullamcorper efficitur. Cras placerat ut turpis pellentesque vulputate. Nam sed consequat tortor. Curabitur finibus sapien dolor. Ut eleifend tellus nec erat pulvinar dignissim. Nam non arcu purus. Vivamus et massa massa.
Vinyl Acetate
Vinyl Acetate is an organic compound with the formula CH₃CO₂CH=CH₂. It's a colorless liquid that serves as a crucial building block for various polymers and other materials.
Key Properties of Vinyl Acetate
- Reactivity: It's highly reactive, making it suitable for polymerization reactions.
- Odor: It has a distinctive, sweet odor.
- Flammability: It's a flammable liquid.
Applications of Vinyl Acetate
Vinyl acetate is used to produce a wide range of products, including:
- Polyvinyl Acetate (PVA): A versatile polymer used in adhesives, paints, and coatings.
- Vinyl Acetate-Ethylene Copolymers (EVA): Flexible polymers used in packaging films, footwear, and hot-melt adhesives.
- Polyvinyl Alcohol (PVA): A water-soluble polymer used in various applications, including textile sizing, paper coatings, and adhesives.
Safety Considerations
While vinyl acetate is a valuable industrial chemical, it's important to handle it with care. It is flammable and can irritate the skin, eyes, and respiratory tract.
Always follow safety guidelines and use appropriate protective equipment when working with vinyl acetate.
Vinyl acetate monomer (VAM)
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Vestibulum sagittis orci ac odio dictum tincidunt. Donec ut metus leo. Class aptent taciti sociosqu ad litora torquent per conubia nostra, per inceptos himenaeos. Sed luctus, dui eu sagittis sodales, nulla nibh sagittis augue, vel porttitor diam enim non metus. Vestibulum aliquam augue neque. Phasellus tincidunt odio eget ullamcorper efficitur. Cras placerat ut turpis pellentesque vulputate. Nam sed consequat tortor. Curabitur finibus sapien dolor. Ut eleifend tellus nec erat pulvinar dignissim. Nam non arcu purus. Vivamus et massa massa.
Vulcanizing Agents
Vulcanizing Agents is a chemical process that converts raw rubber into a harder, more elastic, and more stable material. This process is accomplished by creating crosslinks between the polymer chains of the rubber, giving the final product desirable properties such as resistance to heat, abrasion, and chemicals.
Vulcanizing agents are the substances that create these crosslinks and act as catalysts in this reaction. Sulfur is the most common vulcanizing agent, but there are others, each with their own properties and applications.
Sulfur is known as the primary vulcanizing agent and is widely used in the rubber industry due to its affordability and ease of use. In the sulfur vulcanization process, sulfur atoms are attached to the polymer chains of the rubber, forming a three-dimensional network.
Advantages of using non-sulfur vulcanizing agentsMore speed: Some non-sulfur vulcanizing agents accelerate the vulcanization process. Better properties: Using different vulcanizing agents can improve certain properties such as heat resistance, chemical resistance, and aging resistance. Compatibility with rubber types: Non-sulfur vulcanizing agents can be used for different types of rubber.
Applications of vulcanized productsVulcanized products have a wide range of applications, including: Car tires: Tires are one of the most important vulcanized products designed to support the weight of the vehicle and create friction with the road surface. Hoses and pipes: Rubber hoses and pipes are used to transport various fluids and must be resistant to pressure and chemicals. Flooring: Rubber flooring is used in high-traffic areas such as factories and sports halls due to its resistance to abrasion and slippage. Gaskets and O-rings: Rubber gaskets and O-rings are used to create seals in various systems.
Vulcanizing Boiler
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Vestibulum sagittis orci ac odio dictum tincidunt. Donec ut metus leo. Class aptent taciti sociosqu ad litora torquent per conubia nostra, per inceptos himenaeos. Sed luctus, dui eu sagittis sodales, nulla nibh sagittis augue, vel porttitor diam enim non metus. Vestibulum aliquam augue neque. Phasellus tincidunt odio eget ullamcorper efficitur. Cras placerat ut turpis pellentesque vulputate. Nam sed consequat tortor. Curabitur finibus sapien dolor. Ut eleifend tellus nec erat pulvinar dignissim. Nam non arcu purus. Vivamus et massa massa.
Water Cloth Wrapping & Taking-off …
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Vestibulum sagittis orci ac odio dictum tincidunt. Donec ut metus leo. Class aptent taciti sociosqu ad litora torquent per conubia nostra, per inceptos himenaeos. Sed luctus, dui eu sagittis sodales, nulla nibh sagittis augue, vel porttitor diam enim non metus. Vestibulum aliquam augue neque. Phasellus tincidunt odio eget ullamcorper efficitur. Cras placerat ut turpis pellentesque vulputate. Nam sed consequat tortor. Curabitur finibus sapien dolor. Ut eleifend tellus nec erat pulvinar dignissim. Nam non arcu purus. Vivamus et massa massa.
Wetting Agents (Surfactants)
Wetting agents are a type of surfactant (surface-active agent) that reduce the surface tension of a liquid, allowing it to spread more easily and completely over a surface.
How do they work?
Surface Tension: Liquids naturally tend to minimize their surface area due to cohesive forces between molecules. This results in a phenomenon called surface tension, where the liquid tends to form droplets and resist spreading.
Role of Surfactants: Surfactants have a unique molecular structure with two distinct parts:
Hydrophilic (water-loving) head: This part of the molecule is attracted to water.
Hydrophobic (water-hating) tail: This part of the molecule is repelled by water and attracted to non-polar substances.
Reducing Surface Tension: When a surfactant is added to a liquid, it concentrates at the interface between the liquid and another substance (like a solid surface). The hydrophilic heads interact with the liquid, while the hydrophobic tails interact with the surface. This disrupts the cohesive forces between the liquid molecules, reducing surface tension and allowing the liquid to spread more easily.
Key Properties of Wetting AgentsReduced Surface Tension: This is the primary function of wetting agents. Improved Wetting: Enables liquids to spread more evenly and completely over a surface. Increased Penetration: Allows liquids to penetrate more deeply into porous materials. Improved Dispersion: Helps to disperse solids or other substances more effectively in liquids.
Applications of Wetting AgentsAgriculture: Improve the effectiveness of pesticides and herbicides by ensuring even coverage on plant surfaces. Enhance the efficiency of irrigation by improving water penetration into the soil. Industry: Improve the wetting of pigments in paints and coatings. Enhance the penetration of cleaning solutions into surfaces. Aid in the processing of textiles and paper. Personal Care: Used in shampoos and conditioners to improve wettability of hair. Found in some skin care products to improve the absorption of other ingredients. Examples of Wetting Agents: Surfactants: Soaps, detergents, emulsifiers Fatty acids: Oleic acid, stearic acid Alcohols: Ethanol, isopropanol
Wheel Covers
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Vestibulum sagittis orci ac odio dictum tincidunt. Donec ut metus leo. Class aptent taciti sociosqu ad litora torquent per conubia nostra, per inceptos himenaeos. Sed luctus, dui eu sagittis sodales, nulla nibh sagittis augue, vel porttitor diam enim non metus. Vestibulum aliquam augue neque. Phasellus tincidunt odio eget ullamcorper efficitur. Cras placerat ut turpis pellentesque vulputate. Nam sed consequat tortor. Curabitur finibus sapien dolor. Ut eleifend tellus nec erat pulvinar dignissim. Nam non arcu purus. Vivamus et massa massa.