Hydroxypropyl Methyl Cellulose HPMC
Structure and PropertiesStructure: HPMC is a linear polymer made up of repeating glucose units. Hydroxypropyl and methyl groups are attached to this polymer, giving it viscoelastic properties. Physical Properties: HPMC is a white or slightly yellow powder, odorless and tasteless. It dissolves in cold water to form viscous solutions. The viscosity of these solutions decreases with increasing temperature. Chemical Properties: HPMC is a stable polymer and is resistant to heat, light and many chemicals.
HPMC Applications
- Pharmaceutical Industries
- Food Industries
- Cosmetics Industries
- Construction Industries
Hydroxyquinoline Sulfate
Hydroxyquinoline sulfate is a chemical compound that has been used for various purposes, particularly in the medical and agricultural fields. It is a salt of 8-hydroxyquinoline and sulfuric acid.
- Antimicrobial and Antifungal Properties:
- It has potent antimicrobial and antifungal properties, making it effective in treating skin infections and fungal diseases.
- It's often used in topical ointments and creams to treat skin infections.
- Chelating Agent:
- It can bind to metal ions, making it useful in various applications, including water treatment and metal purification.
- Analytical Chemistry:
- It's used as a reagent in analytical chemistry for the detection and determination of certain metals.
- Agriculture:
- It's used as a fungicide to protect plants from fungal diseases.
Important Considerations:
While hydroxyquinoline sulfate has several beneficial properties, it's important to use it with caution, as it can have potential side effects, particularly when used topically. It's advisable to consult with a healthcare professional before using products containing hydroxyquinoline sulfate.
Industrial Tires
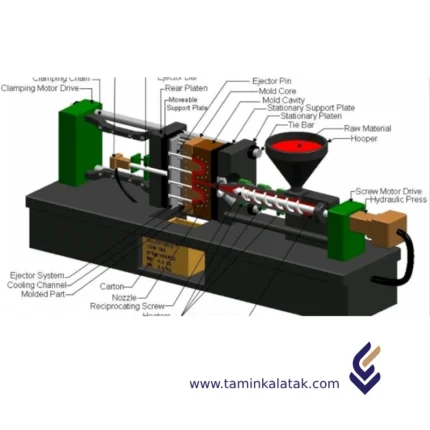
Injection Molding
Types of injection moldingGas-Assisted Injection Molding This process involves injecting gas (commonly nitrogen) into the molten polymer during molding. The gas pushes the molten plastic against the mold walls, creating hollow sections or reducing the amount of material used. Thin-Wall Injection Molding This method Focuses on producing parts with very thin walls, typically less than 1 mm. This requires specialized molds and machines capable of handling high pressures and fast cycle times. Liquid Silicone Rubber (LSR) Injection Molding This method is Used exclusively for liquid silicone rubber (LSR), a thermosetting material that cures when heated. LSR is injected into a heated mold, where it solidifies into a flexible and durable part. Structural Foam Molding A process where a foaming agent or gas is added to the polymer to create parts with a cellular core and solid outer skin. This reduces density and weight while maintaining strength. Metal Injection Molding (MIM) A process that combines metal powders with a polymer binder to create a feedstock. The feedstock is injected into a mold, then the binder is removed, and the part is sintered to achieve a dense metal component.
Advantages of injection molding
- Injection molding is incredibly cost-effective, especially in high-volume applications where thousands to tens of thousands of parts are printed in a workday.
- Injection molding offers many different materials, both general use, and specialty.
- Injection molding provides immense design freedom to product developers and is only held back by mold design, material specifications, and cost.
- Injection-molded parts can be as small as a grain of rice (or smaller) or can be as large as a car dashboard, depending upon the specific type of injection molding being used.
- Injection molding can produce highly complex parts that would otherwise be too time-consuming or difficult to produce with traditional subtractive manufacturing methods.
- Injection molding is a low/no waste manufacturing method, and waste can typically be 100% recycled and reground into stock material for a new injection mold.
Disadvantages of injection molding
- High initial tooling and equipment costs.
- Long lead times for mold design and production.
- Material limitations and risk of defects.
- Environmental and sustainability concerns.
- Design constraints requiring engineering expertise.
- Best suited for high-volume production.
Applications of injection moldingPlastic injection molding is used throughout industry as a means of manufacturing plastic parts in high volumes. Its applications are theoretically endless given the various types of injection molding available combined with its popularity. Still, there are some core usages for which the injection molding process particularly excels. Examples of injection molding applications include (but are not limited to):
- Automotive components
- Food and Beverage packaging
- Stock materials (spools, bar, tube, etc.)
- Toys and figurines
- Furniture components
- Fixtures and fasteners
- Mechanical components (gears, valves, pumps, linkages, etc.)
- Electronic hardware and housings
- Medical device components
- General plastic parts
Inner Tube Splicer
Inner Tube Vulcanizer & Mould
Inner Tubes
Internal Tire Buffing Machine,
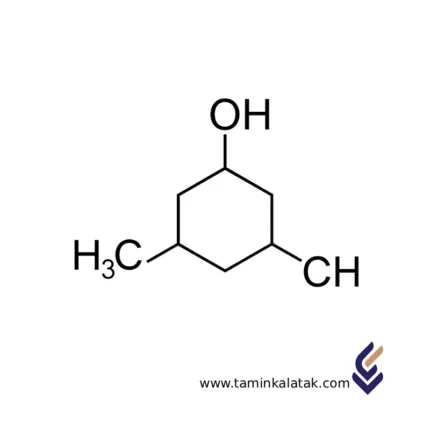
Iso Butanol (Isobutyl Alcohol)
Isobutanol (Isobutyl Alcohol) is a branched primary alcohol containing four carbon atoms. It is widely used across various industries as a solvent, chemical intermediate, fuel additive, and plasticizer. This compound is a colorless liquid with a characteristic alcoholic odor and moderate volatility, belonging to the family of light aliphatic alcohols.
Chemical Structure of Isobutanol
-
Molecular formula: C₄H₁₀O
-
IUPAC name: 2-Methyl-1-propanol
-
Structural formula: (CH₃)₂CHCH₂OH
-
Type of alcohol: Branched primary alcohol
The branched structure of isobutanol (or isobutyl alcohol) distinguishes it from n-butanol, resulting in differences in physical properties, solubility, odor, and chemical reactivity.
Physical and Chemical Properties of Isobutanol
| Property | Description |
|---|---|
| Molecular weight | 74.12 g/mol |
| Odor | Sweet, slightly musty |
| Flash point | ~28 °C (depending on pressure) |
| Solubility in organic solvents | Miscible with alcohols, esters, ethers, chloroform, and benzene |
| Polarity | Moderate – contains a polar hydroxyl group and a short hydrocarbon chain |
| Volatility | Medium – evaporates slower than methanol but faster than ethanol |
| Chemical stability | Stable under normal conditions; unstable with strong acids or oxidizing agents |
| Miscibility | Completely miscible with most organic solvents |
Applications of Isobutanol
1. Industrial Solvent
-
Used in lacquers, resins, adhesives, and solvent-based paints
-
Effective solvent for printing inks, metal coatings, and automotive varnishes
2. Fuel Additive
-
Improves octane rating in gasoline
-
Reduces engine deposits and enhances combustion efficiency
-
Considered a bio-based alternative to methanol in blended biofuels
3. Chemical Synthesis
-
Intermediate in the production of esters, plasticizers, and surfactants
-
Used in the manufacture of isobutyl acetate, butyl acrylate, and various aromatic compounds
4. Oil, Gas, and Agricultural Industries
-
Used as a formulation agent in pesticides, lubricating additives, and extraction agents
-
Applied in oil refining and organic phase separation processes
Advantages of Isobutanol
-
Excellent solvent power for both polar and nonpolar compounds
-
Moderate evaporation rate, providing better control compared to lighter alcohols
-
High efficiency in the synthesis of esters and fragrance compounds
-
High octane number, suitable for blending with fossil fuels
-
Good chemical and environmental stability
Disadvantages of Isobutanol
-
Highly flammable, requiring careful handling and storage
-
Moderate toxicity – may cause respiratory or skin irritation with repeated exposure
-
Limited solubility in water, requiring emulsifiers or mechanical agitation for blending
-
Often more expensive than n-butanol in certain markets
Safety Information for Isobutanol
-
Chemical name: Isobutanol (Isobutyl Alcohol)
-
Chemical formula: C₄H₁₀O
-
CAS Number: 78-83-1
Chemical and Physical Hazards of Isobutanol
| Hazard Type | Description |
|---|---|
| High flammability | Isobutanol has a flash point of around 28 °C. Vapors can ignite easily when exposed to open flames or sparks. |
| Skin and eye irritation | Direct contact may cause skin dryness, irritation, or eye discomfort. |
| Respiratory hazard | Inhalation of vapors in confined spaces may cause dizziness, headache, nausea, or respiratory irritation. |
| Nervous system effects | Prolonged or repeated exposure may adversely affect the central nervous system. |
Safety Measures for Handling Isobutanol
General Precautions
-
Work only in well-ventilated areas
-
Use an organic vapor respirator in industrial environments
-
Wear chemical-resistant gloves (nitrile or PVC), safety goggles, and a lab coat
-
Avoid prolonged or repeated skin contact
In Case of Leakage
-
Absorb spills using non-combustible absorbents such as vermiculite or sand
-
Ventilate the area and remove all ignition sources
-
Use anti-static, spark-proof tools for cleanup
In Case of Fire
-
Use alcohol-resistant foam, CO₂, or dry chemical extinguishers
-
Avoid using high-pressure water streams (may spread the fire)
-
Firefighting personnel should wear protective gear and alcohol-resistant suits
Storage Conditions for Isobutanol
| Parameter | Recommendation |
|---|---|
| Storage temperature | 5 – 30 °C – avoid extreme cold or direct heat |
| Storage containers | Use metal drums or high-density polyethylene (HDPE) containers resistant to solvents |
| Ventilation | Store in a well-ventilated warehouse, free from heat sources |
| Labeling | Containers should be clearly labeled with flammable and irritant hazard symbols |
| Incompatible materials | Keep away from strong oxidizers, concentrated acids, and chlorates |
Isobutyl Acetate
Isobutyl Acetate is an organic compound with the chemical formula C₆H₁₂O₂. It is a colorless liquid with a distinctive fruity odor, often described as similar to banana or pear.
Properties of Isobutyl Acetate
- Odor: Fruity, pleasant odor
- Solubility: Slightly soluble in water but miscible with most organic solvents
- Flammability: Highly flammable
- Toxicity: Low toxicity, but can cause irritation to skin, eyes, and respiratory tract
Applications of Isobutyl Acetate
Isobutyl acetate is a versatile compound with a wide range of applications:
-
Solvent:
- Paints and Coatings: Used as a solvent in various paints, varnishes, and lacquers.
- Adhesives: Employed as a solvent in adhesives and sealants.
- Cleaning Products: Utilized in cleaning solutions and degreasers.
-
Flavoring Agent:
- Its fruity odor makes it a popular flavoring agent in food and beverages.
- Used in the production of artificial fruit flavors.
-
Perfumery:
- Employed as a fragrance ingredient in perfumes and colognes.
-
Chemical Intermediate:
- Used as a starting material for the synthesis of other chemicals.
Safety Considerations
While isobutyl acetate is a useful compound, it's important to handle it with care due to its flammability and potential health hazards. Always use it in well-ventilated areas and avoid contact with skin, eyes, and inhalation of its vapors.
Isoprene/PP Reclaimed Rubber
Applications of recycled isoprene/PP rubberAutomotive industry: production of gaskets, seat covers, engine parts and hoses. Construction industry: production of insulation, coatings and sealants. Consumer goods industry: production of toys, household appliances and sporting goods. Shoe industry: production of shoe soles and uppers.
Advantages of using recycled isoprene/PP rubberCost reduction: Using recycled materials reduces production costs. Environmental protection: Reduction of rubber waste and reduction of raw material consumption. Suitable mechanical properties: A combination of the properties of natural rubber and plastic. Recyclability: This material can also be recycled.
Isopropyl Acetate
Applications of Isopropyl AcetateIsopropyl acetate is used in various industries due to its unique properties: Solvent: Paints and Coatings: Used as a solvent in a variety of paints, varnishes, and lacquers. Adhesives: Used as a solvent in adhesives and sealants. Cleaning Products: Used in cleaning and degreasing solutions. Flavoring: Its fruity odor has made it a popular flavoring in foods and beverages. Used in the production of artificial fruit flavors.
Chemical IntermediatesUsed as a starting material for the synthesis of other chemicals. Safety Considerations While isopropyl acetate is a useful compound, it should be used with caution due to its flammability and potential health hazards. Always use it in well-ventilated areas and avoid contact with skin, eyes, and inhalation of its vapors.