Ethoxylated stearic acid
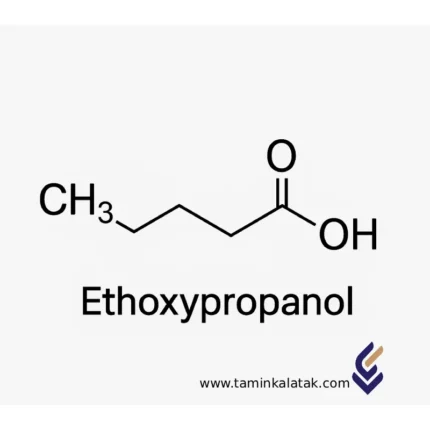
EthoxyPropanal
Characteristics
- Appearance: Colorless liquid
- Odor: Mild, ether-like smell
- Chemical Formula: C₅H₁₀O₂
- Solubility: Soluble in water and most organic solvents
Applications Ethoxypropanal
- Industrial Applications: Used as a solvent in the production of paints, coatings, leather sealants, wood stains, furniture polishes, inks, and cleaning agents.
- Additives: Can be used as an additive in adhesives, agrochemicals, and nail care products.
Properties
- Boiling Point: Approximately 130°C (266°F)
- Density: Varies depending on the specific isomer
Ethoxypropanol
Ethoxypropanol (1-Ethoxy-2-propanol) is a clear, colorless solvent with a mild ether-like odor. Owing to the presence of both polar (–OH) and non-polar (–O–CH₂CH₃) functional groups, this compound belongs to the class of mono-functional glycol ethers.
It is widely used in paint, coating, printing, detergent, resin, and cosmetic formulations as an effective solvent with controlled evaporation rate and excellent solubility characteristics.
Chemical Structure of Ethoxypropanol
-
Molecular formula: C₅H₁₂O₂
-
Structural formula: CH₃–CH(OCH₂CH₃)–CH₂–OH
-
IUPAC name: 1-Ethoxy-2-propanol
-
Chemical type: Mono-functional glycol ether containing both a hydroxyl group (–OH) and an ethoxy branch (–O–CH₂CH₃), suitable for dissolving both polar and non-polar compounds
Physical and Chemical Properties
| Property | Description |
|---|---|
| Appearance | Colorless to very pale yellow liquid with an ether-like odor |
| Boiling point | ~132 °C |
| Refractive index | 1.4065–1.407 |
| Flash point | 38.5–42 °C (closed cup) |
Applications of Ethoxypropanol
1. Paint and Resin Industry
-
Used as a medium-evaporation solvent in water-based and solvent-based paints and coatings
-
Improves flow, leveling, and surface gloss of coatings
-
Reduces surface tension, enhancing adhesion of paints to various substrates
2. Printing and Ink Industry
-
Serves as a pigment carrier and solvent in inkjet, screen, flexographic, and gravure inks
-
Controls drying speed and prevents nozzle clogging, especially in thermal inkjet printers
-
Enhances print sharpness and prevents pigment sedimentation
3. Detergents and Industrial Cleaners
-
Used as a solvent for degreasing metal, glass, and plastic surfaces
-
Ideal for semi-volatile, non-corrosive cleaners due to its mild odor and relatively low toxicity
-
An effective, environmentally friendly alternative to heavier glycol ethers
4. Cosmetics and Personal Care (Limited, Regulated Use)
-
Acts as a carrier or co-solvent for aromatic or active ingredients in certain skin and hair formulations
-
Usage is restricted due to potential skin irritation at high concentrations
-
Must comply with IFRA and REACH regulatory standards
5. Agricultural and Pesticide Formulations
-
Enhances dispersion and solubility of active ingredients in spray formulations
-
Improves foliar absorption and reduces surface tension in liquid fertilizer and pesticide systems
Advantages of Ethoxypropanol
-
Dual solubility: Efficiently dissolves both polar and non-polar compounds
-
Moderate evaporation rate: Allows controlled drying in coating and ink applications
-
Excellent compatibility with both water and organic solvents
-
Chemical and physical stability in complex formulations
-
Surface tension reduction and improved lubricity in chemical systems
Limitations and Disadvantages
-
Flammable – requires proper ventilation and storage away from heat sources
-
Irritating to skin and eyes upon direct contact
-
Vapor inhalation at high concentrations may cause respiratory or neurological effects – must comply with OSHA exposure limits
-
Requires high-purity grades for cosmetic or pharmaceutical applications
Safety and Storage Guidelines
From a safety perspective, Ethoxypropanol should be handled and stored with care, as its vapors can irritate the eyes, skin, and respiratory system. Prolonged skin exposure may lead to dryness or inflammation.
Precautions and Storage Conditions
-
Store in a cool, dry, and well-ventilated area.
-
Keep away from open flames and ignition sources (highly flammable).
-
Use chemical-resistant gloves, safety goggles, and a respirator when handling.
-
Containers should be tightly closed, solvent-resistant, and clearly labeled to prevent vapor leakage and evaporation.
Ethyl Acetate
Characteristics Ethyl AcetateAppearance: Colorless liquid Odor: Fruity characteristic smell, commonly recognized in glues and nail polish removers Flammability: Extremely flammable with a flashpoint of -4°C, has a flammability rating of 3 Solubility: Highly miscible with all common organic solvents (alcohols, ketones, glycols, esters), but only slightly miscible in water
ethyl acetate applicationsIndustrial Applications: Used as a solvent in paints, coatings, adhesives, and inks Cosmetic Industry: Commonly found in nail polish removers and other cosmetic formulations Food Industry: Sometimes used as a flavoring agent due to its fruity aroma
Ethyl acrylate
Ethylene Glycol
Physical and chemical propertiesSolubility: It dissolves well in water and many organic solvents. Boiling point: It is relatively high. Viscosity: It has a moderate viscosity. Density: It is slightly higher than water. Antifreeze property: Ethylene glycol greatly lowers the freezing point of water and for this reason is used as an antifreeze in cooling systems.
Applications of Ethylene GlycolAntifreeze: The main use of ethylene glycol is as an antifreeze in the cooling systems of automobiles, engines, and industrial equipment. Production of polyethylene terephthalate (PET): One of the most important uses of ethylene glycol is in the production of polyethylene terephthalate (PET), which is used to make beverage bottles, synthetic fibers, and packaging films. Solvent: Ethylene glycol is used as a solvent for paints, inks, and resins. Antifreeze in the food industry: It is used as an antifreeze for frozen foods in some food industries. Production of explosives: In the past, ethylene glycol was used in the production of explosives, but due to its dangers, this application has received less attention. Advantages of using ethylene glycol Strong antifreeze property: Effectively lowers the freezing point of water. Good solubility: Suitable as a solvent for many chemicals. Mass production and reasonable price: Due to mass production, the price of ethylene glycol is relatively reasonable.
Hazards and Safety PrecautionsToxicity: Ethylene glycol is a toxic substance and ingestion can cause severe poisoning and even death. Irritability: Ethylene glycol contact with skin or eyes can cause irritation. Flammability: Ethylene glycol vapors are flammable. Environmental: Ethylene glycol discharge into the environment can cause water and soil contamination.
Ethylene Glycol Monostearate
PropertiesAppearance: White powder or liquid Odor: Slight estery odor Solubility: Soluble in water and alcohol, insoluble in oil Physical properties: Used as a brightening agent in cosmetic creams and lotions
Applications Ethylene Glycol MonostearateCosmetic industries: Creams and lotions: EGMS is used as an emollient and brightening agent in creams and lotions, and gives a soft and smooth feeling to the skin. Shampoos and soaps: Used as an emulsifier and foam stabilizer in the formulation of shampoos and soaps. Food industry: Food additives: Used as an emulsifier in food production to help improve the texture and uniformity of products. Pharmaceutical industry: Pharmaceutical formulations: EGMS is used as an additive in the production of drugs and dietary supplements.
Chemical and industrial industriesPaints and coatings: Used as an emulsifier and stabilizer in the production of industrial paints and coatings. Adhesives and lubricants: Used as an additive in the formulation of adhesives and lubricants to improve the performance and stability of these products.
Ethylene Propylene Diene Monomer (EPDM) Reclaimed Rubber
Key CharacteristicsComposition: EPDM is a terpolymer of ethylene, propylene, and a small amount of a diene monomer (typically dicyclopentadiene or ethylidene norbornene). Properties: Excellent Weather Resistance: Highly resistant to ozone, sunlight, and weathering. Good Chemical Resistance: Resistant to many chemicals, including acids, alkalis, and oils. Good Heat Resistance: Can withstand high temperatures without significant degradation. Excellent Electrical Insulation: Exhibits good electrical insulating properties. Good Flexibility and Durability: Remains flexible over a wide temperature range.
Applications EPDMAutomotive: Seals, gaskets, hoses, weatherstripping Construction: Roofing membranes, waterproofing sheets, geomembranes Wire and Cable: Insulation for wires and cables Industrial: Chemical hoses, gaskets, diaphragms Consumer Goods: Sporting goods, toys
Advantages of EPDMLong Lifespan: High resistance to weathering and aging, leading to extended service life. Versatility: Suitable for a wide range of applications due to its diverse properties. Environmentally Friendly: Some grades of EPDM can be recycled. Limitations: Lower Tensile Strength: Compared to some other rubbers, EPDM may have lower tensile strength. Limited Oil Resistance: While resistant to many chemicals, it may exhibit limited resistance to certain oils and solvents.
Ethylene TetraFluoroEthylene (ETFE)
StructureEthylene Tetrafluoroethylene (ETFE) is a copolymer composed of ethylene (C₂H₄) and tetrafluoroethylene (C₂F₄) units. Its molecular structure consists of a repeating chain of carbon atoms bonded to both fluorine and hydrogen atoms, giving it a unique combination of chemical resistance, mechanical strength, and thermal stability. The presence of fluorine atoms enhances its non-stick properties and high resistance to UV radiation, while the ethylene component contributes to its flexibility and toughness. Unlike polytetrafluoroethylene (PTFE), ETFE has a lower fluorine content, making it slightly less chemically inert but significantly stronger and more impact-resistant. This structural composition results in a lightweight, durable material that retains its transparency and mechanical properties even under extreme environmental conditions. ETFE's semi-crystalline structure also allows it to be processed into thin films, making it highly suitable for architectural applications, insulation, and protective coatings.
PropertiesEthylene Tetrafluoroethylene (ETFE) possesses a unique combination of properties that make it highly versatile across various applications. It is exceptionally lightweight, weighing only about 1% of the weight of glass, while maintaining high tensile strength and impact resistance. Its chemical structure provides outstanding resistance to ultraviolet (UV) radiation, weathering, and most chemicals, ensuring long-term durability in harsh environments. ETFE is highly transparent, allowing up to 95% of natural light to pass through, making it an excellent choice for architectural applications. Additionally, it has a low coefficient of friction, giving it self-cleaning and anti-fouling properties. The material is also highly flexible, capable of stretching up to three times its original length without losing integrity. With a high melting point of around 265°C (509°F), ETFE exhibits excellent thermal stability and can withstand extreme temperature fluctuations without degradation. Furthermore, it is a recyclable material, adding to its sustainability by reducing environmental impact. These combined properties make ETFE a preferred choice for applications in construction, aerospace, medical, and renewable energy industries.
Advantages
- Lightweight: Weighs only about 1% of the weight of glass.
- High Strength & Durability: Resistant to mechanical stress, impact, and punctures.
- Transparency: Allows up to 95% natural light transmission.
- UV & Weather Resistance: Does not degrade under prolonged sunlight exposure.
- Chemical Resistance: Withstands most acids, solvents, and other harsh chemicals.
- Self-Cleaning Surface: Low friction and non-stick properties prevent dirt accumulation.
- Thermal Stability: Can withstand extreme temperatures (-185°C to 150°C).
- Flexibility & Elasticity: Can stretch up to three times its length without damage.
- Eco-Friendly & Recyclable: Can be melted down and reused.
Disadvantages
- Higher Cost: More expensive than traditional materials like glass or polycarbonate.
- Flammability Concerns: Can burn under extreme conditions but is self-extinguishing.
- Limited Structural Support: Needs additional framing or inflation systems for strength.
- Softness & Scratch Sensitivity: Can be scratched more easily than glass.
- Noise Insulation: Provides less soundproofing compared to solid materials.
Applications
- Architecture & Construction: Used in stadiums, skylights, and domes (e.g., Allianz Arena, Eden Project).
- Aerospace & Automotive: Used for wire insulation and protective coatings.
- Medical Industry: Used for tubing, catheters, and biocompatible coatings.
- Chemical Industry: Lining for pipes and tanks due to its chemical resistance.
- Solar & Renewable Energy: Used in photovoltaic panel coatings and greenhouse covers.
- Electronics: Used in high-performance cable insulation for aerospace and telecommunications.
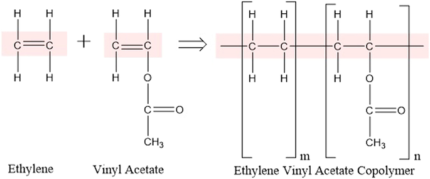
Ethylene Vinyl Acetate/ VAC- copolymers (EVA)
StructureEthylene Vinyl Acetate (EVA) is a copolymer composed of ethylene and vinyl acetate (VAC) monomers, with its structure characterized by randomly distributed vinyl acetate units within a polyethylene-like backbone. The proportion of vinyl acetate in the copolymer significantly influences its properties, with lower vinyl acetate content (typically below 10%) resulting in a more rigid, polyethylene-like material, while higher vinyl acetate content (above 40%) leads to a more rubbery and flexible structure. The presence of vinyl acetate disrupts the crystallinity of polyethylene, enhancing the copolymer’s flexibility, impact resistance, and transparency. EVA exhibits a balance between thermoplastic and elastomeric properties, making it widely used in applications such as adhesives, foams, films, and footwear. Its molecular structure provides excellent toughness, stress-crack resistance, and adhesion to various substrates, making it a versatile polymer in multiple industries.
PropertiesEthylene Vinyl Acetate (EVA) copolymers exhibit a unique combination of properties that vary based on the vinyl acetate (VAC) content. They offer excellent flexibility, elasticity, and toughness, with higher VAC content leading to increased softness, transparency, and impact resistance. EVA has low-temperature resistance, maintaining flexibility even at sub-zero temperatures, and demonstrates good stress-crack resistance. It is also lightweight, has a low density, and provides excellent adhesion to various substrates, making it ideal for adhesives and coatings. Additionally, EVA is resistant to UV radiation and environmental stress, contributing to its durability in outdoor applications. It has good chemical resistance to water, oils, and certain solvents, though it may degrade under high temperatures or prolonged exposure to strong chemicals. The copolymer is also non-toxic, making it suitable for medical and food-contact applications. Its thermal and electrical insulation properties further enhance its versatility in industrial, packaging, and footwear applications.
Applications of Ethylene Vinyl Acetate (EVA) Copolymers:
- Footwear: Used in midsoles, insoles, and outsoles for cushioning and flexibility.
- Adhesives: Hot melt adhesives in packaging, bookbinding, and woodworking.
- Foams: Sports mats, yoga mats, and padding materials.
- Packaging: Film applications for food packaging and medical films.
- Automotive: Interior trims, soundproofing, and under-the-hood components.
- Solar Panels: Encapsulation of photovoltaic cells for durability and insulation.
- Wire & Cable Insulation: Used in electrical applications due to flexibility and insulation properties.
- Toys & Consumer Goods: Soft, flexible materials for safety and durability.
Advantages of EVA Copolymers:
- Flexibility & Softness: Offers rubber-like elasticity and comfort.
- Lightweight: Reduces overall product weight while maintaining durability.
- Good Adhesion: Bonds well with various substrates, making it ideal for adhesives.
- Weather & UV Resistance: Suitable for outdoor applications like solar panels and footwear.
- Chemical Resistance: Resists many chemicals, oils, and solvents.
- Low-Temperature Performance: Maintains flexibility in cold conditions.
- Non-Toxic & Safe: Used in food packaging and medical applications.
Disadvantages of EVA Copolymers:
- Lower Heat Resistance: Can degrade or deform at high temperatures.
- Lower Mechanical Strength: Compared to other thermoplastics like polyethylene or polypropylene.
- Aging & Degradation: May degrade over time when exposed to UV radiation or harsh conditions.
- Flammability: Not inherently flame-retardant without additives.
- Cost: Can be more expensive than some alternative plastics like PVC.
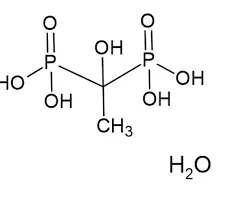
Etidronic acid
Calcium Diacetate is a chemical compound with the formula Ca(CH₃COO)₂. It is found as a white crystalline powder and is usually odorless or has a faint vinegar-like smell. Other names include calcium acetate and calcium ethanoate. The anhydrous form is very hygroscopic, so it is commonly supplied as the monohydrate (Ca(CH₃COO)₂·H₂O).
Structure of Calcium Diacetate
A molecule of calcium diacetate consists of one Ca²⁺ cation and two acetate anions (CH₃COO⁻). The calcium ion serves as the central cation, ionically bonded to two negatively charged acetate groups. Its crystalline structure varies depending on hydration.
Key Properties of Calcium Diacetate
-
Appearance: White crystalline powder
-
Odor: Generally odorless, or faint vinegar smell
-
Solubility: Freely soluble in water; slightly soluble in methanol; insoluble in acetone, ethanol, and benzene
-
Molar Mass: Approximately 158.17 g/mol (anhydrous)
-
Melting/Decomposition Point: Decomposes around 160 °C
-
Density: About 1.509 g/cm³
-
pH of Aqueous Solution: Neutral to slightly alkaline (pH ~6.3–9.6 for 10% solutions)
-
Hygroscopicity: The anhydrous form strongly absorbs moisture
Advantages of Calcium Diacetate
-
Source of Calcium: Used in dietary and pharmaceutical supplements
-
Food Preservative: Added under E263, inhibits mold growth and extends shelf life of baked goods
-
pH Regulator: Acts as a buffering agent in food processing
-
Stabilizer: Used in textile dyeing for color fixation and in food like canned vegetables and tofu to improve texture
-
Generally Recognized as Safe: Considered non-toxic at permitted levels
-
Good Water Solubility: Facilitates incorporation in various formulations
Disadvantages of Calcium Diacetate
-
Digestive Side Effects: Overconsumption or sensitivity may cause mild GI symptoms like bloating, constipation, or gas
-
Hygroscopic Nature: Needs dry storage to prevent moisture absorption
-
Drug Interactions: May interfere with absorption of certain medications—consultation advised when taken concurrently
-
Risk of Hypercalcemia: Excessive intake can raise blood calcium levels, leading to related health risks
Applications of Calcium Diacetate
Food Industry:
-
Preservative (anti-mold) in breads, pastries, and other baked goods
-
pH regulator and buffer
-
Stabilizer/firming agent in foods such as tofu (as a preferred alternative to calcium sulfate) and canned vegetables
-
Ingredient in candies, desserts, puddings
-
Additive in animal feed
Pharmaceutical Sector:
-
Buffering agent in medicinal formulations
-
Calcium supplement for deficiency
-
Phosphate binder for dialysis patients with high blood phosphate
Textile Industry:
-
Used as a color fixer
Chemical Industry:
-
Catalyst in select chemical reactions
-
Previously used in acetone production
Wastewater Treatment:
-
Employed to remove phosphate from wastewater
Soap Production:
-
Utilized as an alkali in certain soap manufacturing
Fire Gel Production:
-
When dissolved in alcohol at saturation, it forms a semi-solid, combustible gel suitable for flame use
Expandable PolyStyrene (EPS)
Expanded Polystyrene (EPS) is a rigid, closed-cell thermoplastic foam material produced from solid polystyrene beads. This polymer is obtained by polymerizing styrene monomer and contains a blowing agent (typically pentane) dissolved within the polystyrene beads.
Each solid bead of polystyrene contains small amounts of this gas, which expands when exposed to heat (in the form of steam), forming a closed-cell foam structure. These expanded cells can occupy up to 40 times the volume of the original polystyrene beads. Through further heat treatment and molding, large EPS blocks can be shaped into custom forms and components for diverse industrial uses.
Structure of Expanded Polystyrene
The structure of EPS consists of small, closed-cell foam beads made from polystyrene. When heated, these beads expand to as much as 50 times their original size. Each bead contains numerous microscopic air cavities that contribute to its lightweight and insulating properties.
Properties of Expanded Polystyrene (EPS)
EPS serves as a core polymer material in many applications due to its lightweight, moisture resistance, and long service life.
Studies show that softening of EPS begins between 100 °C and 120 °C. During thermal degradation, it melts at approximately 160 °C, vaporizes, and at around 275 °C, releases toxic gases.
EPS is an inert, low-density hydrocarbon thermoplastic, consisting of spherical granules that are approximately 2% polystyrene and 98% air.
Applications of Expanded Polystyrene (EPS)
1. Building and Construction
EPS is widely used in construction because of its excellent thermal insulation and lightweight characteristics. It is used as:
-
Insulation panels for façades, walls, roofs, and floors.
-
Buoyant material in marine structures such as marinas and floating bridges.
-
Lightweight fill in road and railway embankments to reduce soil load.
2. Food Packaging
EPS is commonly used in food packaging for products such as seafood, fruits, and vegetables.
It is also used for food-service containers, including drink cups, food trays, and clamshell boxes.
3. Industrial Packaging
EPS provides complete protection for industrial products during handling and transportation, ensuring safety against shock and mechanical damage.
4. Other Applications
EPS can be molded into virtually any shape — for example:
Sport helmets, child car seats, cushioning pads, structural insulated panels (SIPs), and lightweight automotive seating.
Advantages of EPS
✅ Lightweight
✅ Water-resistant
✅ Easy to manufacture
✅ Energy-efficient
✅ Durable and long-lasting
Disadvantages of EPS
❌ Vulnerable to mechanical compression
❌ Limited fire resistance
❌ Non-biodegradable
EPS Market Price in Türkiye
The price of Expanded Polystyrene (EPS) in Türkiye depends on various factors, including market fluctuations, grade type, manufacturer brand, and supply-demand conditions.
For the latest EPS pricing, you can contact our commercial experts to receive up-to-date market rates and guidance on selecting the most cost-effective material.
Purchasing EPS
To purchase EPS, our technical experts can assist you in selecting the appropriate grade to ensure precise and efficient production tailored to your product requirements.
Common EPS Grades
EPS-200 (Snowa Grade)
Snowa EPS-200 is a versatile grade of lightweight expandable polystyrene with medium-to-coarse bead size and high moldability. It is widely used in packaging, insulation, and lightweight ceiling block production.
This grade contains a controlled amount of pentane blowing agent for optimal pre-expansion and molding performance.
F100 EPS
F100 EPS is a specialized grade designed for producing lightweight, insulated, and moldable products across various industries.
Due to its excellent mechanical properties, thermal performance, and high formability, it is ideal for packaging, construction, and industrial components.
EPS-200 (Tabriz Petrochemical)
EPS-200 from Tabriz Petrochemical is a high-density, durable EPS grade used for insulation and structural components.
It features excellent dimensional stability, precise molding capability, and outstanding thermal resistance, making it suitable for construction, industrial packaging, and technical molding applications.
EPS-300 (Tabriz Petrochemical)
Similar to EPS-200, EPS-300 is produced by Tabriz Petrochemical and offers high density, multi-stage pre-expansion capability, and precise molding characteristics.
It is primarily used in construction, heavy-duty industrial packaging, and structural components.
EPS Manufacturing Process
1. Pre-Expansion
Raw EPS beads are exposed to steam, which vaporizes the blowing agent, expanding the beads several times their original volume.
Precise control of temperature and time determines the final foam density.
2. Stabilization
The expanded beads are stored in ventilated silos to allow internal pressure to equalize, ensuring they are ready for molding.
This step is crucial to achieving uniform, defect-free foam.
3. Molding
Pre-expanded beads are placed in molds and reheated with steam. The beads fuse together, forming a solid part in the desired shape and dimensions — such as blocks, sheets, or custom components.
4. Drying and Cutting
After molding, the final product is dried and, if necessary, cut to the required dimensions.
Key Features of EPS
-
Lightweight and excellent thermal insulation → ideal for building insulation (walls, ceilings, floors).
-
Shock absorption and energy dissipation → suitable for protective packaging of sensitive equipment.
-
Easy and economical processing → enables high-volume production at low cost.
-
Recyclable → reduces environmental impact and promotes sustainability.
Market Outlook and Industry Insights
With the continuous growth of the construction and packaging sectors, global and domestic demand for Expanded Polystyrene (EPS) is steadily increasing.
Companies investing in modern production equipment and precise process control can produce EPS with uniform quality meeting international standards.
Summary
Expanded Polystyrene (EPS) is one of the most important lightweight and insulating polymers used in construction, packaging, and technical applications.
Its low weight, excellent thermal and moisture resistance, and easy processability make it a cost-effective and efficient alternative to traditional insulation materials, helping reduce energy consumption and production costs.
EPS is available in several grades — including F100, EPS-200, and EPS-300 — each optimized for specific uses such as ceiling blocks, industrial packaging foam, and thermal insulation panels.
The choice of grade depends on density, cell size, and blowing-agent content, all of which determine the mechanical performance of the final product.
Economically, EPS pricing in Türkiye is influenced by global styrene prices, exchange rates, and supply levels from domestic petrochemical producers such as Tabriz Petrochemical and Qa’ed Basir Petrochemical.
Selecting a reliable supplier and maintaining real-time pricing awareness are key factors for successful production planning.
Tamin Kala Tech Co., leveraging an extensive domestic and international supply network, provides specialized EPS grades for diverse industries.
Our technical support team offers expert consultation on grade selection, processing conditions, and production optimization to help improve your product quality and operational efficiency.