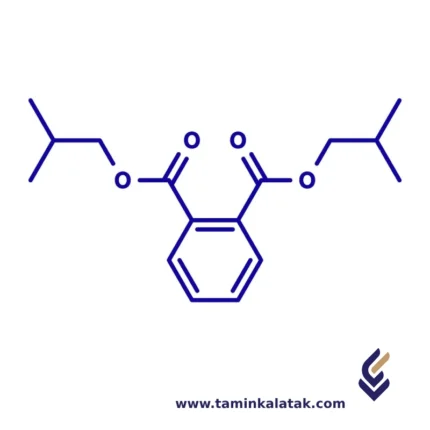
DiIsoButyl Phthalate (DIBP)
Diisobutyl Phthalate (DIBP) is a phthalate ester used primarily as a plasticizer. It is similar in structure and function to dibutyl phthalate (DBP) but has isobutyl groups instead of normal butyl groups.
StructureDiisobutyl phthalate (DIBP) has a chemical structure consisting of a benzene ring with two ester functional groups attached at the 1,2-positions. These ester groups are derived from isobutanol, meaning each ester group contains a branched isobutyl (-CH₂CH(CH₃)₂) moiety. The core structure is based on phthalic acid, where the carboxyl groups are esterified with isobutyl alcohol. This results in a molecular framework that maintains the flexibility and plasticizing properties characteristic of phthalates. The branched nature of the isobutyl groups influences its solubility and interaction with polymers, making it effective in softening plastic materials.
PropertiesDiisobutyl phthalate (DIBP) is a colorless to slightly yellowish liquid with a faint odor. It has a molecular weight of 278.35 g/mol and a dibp chemical formula of C₁₆H₂₂O₄. It is insoluble in water but highly soluble in organic solvents such as ethanol, acetone, and benzene. DIBP has a boiling point of approximately 327°C and a low vapor pressure, making it relatively stable under normal conditions. It functions as a plasticizer by reducing the brittleness of polymers, improving flexibility and workability. Its branched isobutyl groups contribute to its lower viscosity compared to other phthalates. DIBP has good compatibility with cellulose-based plastics, rubber, adhesives, and coatings. However, due to concerns about its potential reproductive toxicity and endocrine-disrupting properties, its use is regulated in certain regions.
Applications
- Used as a plasticizer in plastics, resins, and rubber to enhance flexibility and durability.
- Found in adhesives, sealants, and coatings to improve elasticity and workability.
- Utilized in the production of cellulose-based plastics and nitrocellulose lacquers.
- Commonly used in printing inks and paints to improve film-forming properties.
- Sometimes found in cosmetics and personal care products, though its use is now restricted in many regions.
Advantages
- Provides effective plasticization, improving flexibility and softness of materials.
- Offers good compatibility with various polymers, especially cellulose-based plastics.
- Enhances durability and longevity of coatings, adhesives, and inks.
- Low-cost alternative to other plasticizers with similar performance.
Disadvantages
- Classified as a Substance of Very High Concern (SVHC) due to reproductive toxicity.
- Restricted under REACH regulations in the EU and other regulatory frameworks.
- Potential endocrine-disrupting effects raise health and environmental concerns.
- Limited water solubility can lead to persistence in the environment.
- Replacement with safer alternatives is increasingly being encouraged.
Dipropylene Glycol
Dipropylene Glycol (DPG) is an organic chemical compound that occurs as a colorless, almost odorless, and viscous liquid. This substance is widely used in various industries due to its special physical and chemical properties.
Chemical structure and physical propertiesStructure: Dipropylene glycol is actually a mixture of three isomers, each of which has two hydroxyl (OH) groups attached to a propylene chain. Physical properties: Solubility: Miscible in water and many organic solvents. Viscosity: It has a high viscosity, which gives it a softening property. Boiling point: It has a high boiling point, which makes it stable at high temperatures. Toxicity: It has low toxicity, which is why it is used in many consumer products.
Dipropylene Glycol ApplicationsCosmetics: Emollient: Used in lotions, creams, and other cosmetic products due to its emollient properties. Solvent: Used to dissolve pigments and other ingredients in cosmetic products. Fragrance carrier: Used as a fragrance carrier in perfumes and colognes. Food industry: Solvent: Used in the production of food extracts and flavors. Emollient: Used to maintain moisture in some food products. Paint and coating industry: Solvent: Used in the production of industrial paints and coatings. Emollient: Used to improve the flexibility of coatings. Pharmaceutical industry: Solvent: Used as a solvent in the production of some drugs. Emollient: Used to improve the texture of some pharmaceutical products. Textile industry: Emollient: Used to soften textile fibers. Hydraulic systems: Used as a hydraulic fluid in some systems. Advantages of using dipropylene glycol High solubility: Miscible in many solvents. Suitable viscosity: Acts as a softener and lubricant. Low toxicity: Less toxic than many other solvents. Odorless: Odorless and does not create an unpleasant odor in the final products. Safety notes Skin and eye contact: Contact with skin and eyes can cause irritation. Inhalation: Inhalation of its vapors can cause irritation of the respiratory tract. Flammable: Flammable at high temperatures. Gloves, safety glasses, and a mask should be worn when working with dipropylene glycol. Also, the workplace should be properly ventilated.
Disodium Lauryl Ether Sulfosuccinate
Disodium Lauryl Ether Sulfosuccinate is a common surfactant used in various personal care products, particularly in shampoos, body washes, and facial cleansers. It's known for its mild cleansing properties and ability to create a rich lather.
Properties of Disodium Lauryl Ether SulfosuccinateMildness: DLSS is considered a mild surfactant, making it suitable for sensitive skin. Foaming: It has excellent foaming properties, contributing to the lathering action of many cleansing products. Solubility: It is highly soluble in water. Biodegradability: It is generally considered biodegradable.
Uses of Disodium Lauryl Ether SulfosuccinateShampoos: DLSS is often used in shampoos to gently cleanse hair without stripping away natural oils. Body washes: It's a common ingredient in body washes due to its mild cleansing properties. Facial cleansers: DLSS is used in facial cleansers to remove dirt and oil without irritating the skin. Other personal care products: It can be found in various other products like bubble baths, hand soaps, and baby shampoos.
Safety ConsiderationsDLSS is generally considered safe for use in personal care products. It is not known to cause significant skin irritation or allergic reactions. However, as with any cosmetic ingredient, individual sensitivities may vary. If you have sensitive skin, it's always a good idea to patch test new products before using them on a larger area.
Disodium Phosphate
Disodium Phosphate is a chemical compound with the chemical formula Na₂HPO₄. This substance is in the form of a white crystalline powder and has wide applications in various industries due to its unique properties.
Properties of Disodium PhosphateHigh solubility in water: It dissolves easily in water and creates alkaline solutions. Buffering property: It can reduce pH changes in solutions. Phosphorus source: It contains a large amount of phosphorus, which is essential for many biological processes.
Disodium Phosphate applicationsFood industry: PH regulator: It is used to adjust the pH in food products such as cheese, soft drinks, and processed meats. Emulsifying agent: It acts as an emulsifier in the production of dairy products and ice cream. Anti-caking agent: It is used in washing powders and other powdered products. Pharmaceutical Industry: Drug Carrier: Used as a drug carrier in some formulations. PH Regulator: Used to adjust the pH in pharmaceutical products. Water and Wastewater Industry: Water Softener: Used to soften hard water. Water Treatment: Used as a coagulant in water treatment. Other Industries: Textile Industry: Used as a softening and anti-wrinkle agent in the textile industry. Paper Industry: Used as a quality improvement agent in paper production.
Advantages of Using Disodium PhosphateHigh Solubility: Easily dissolves in formulations. Buffering Property: Helps maintain pH stability. Source of Phosphorus: Essential for many biological processes. Wide Applications: Used in various industries. Important Points in Using Disodium Phosphate Skin and Eye Contact: Causes irritation if contacted with skin and eyes. Inhalation: Inhalation of dust from this material can cause respiratory problems. Storage: Store in closed containers in a cool, dry place.
Dispersants & Emulsifiers
Dispersants and emulsifiers are substances used to stabilize mixtures and improve the separation of particles in various applications. They are essential in industries such as food, pharmaceuticals, cosmetics, and lubricants.
Dispersants are additives that help to keep particles suspended in a liquid, preventing them from settling or clumping together. They are commonly used in paints, coatings, and industrial processes.
- Water treatment
- paints
- coatings
- concrete formulations
- Food products
- cosmetics
- pharmaceuticals
Dodecyl Phenol
Dodecylphenol is an organic compound that has been widely used in various industrial applications. However, due to its persistent nature and potential environmental and health risks, its use has been significantly restricted in many countries.
Structure and PropertiesDodecylphenol is a phenolic compound with a 12-carbon alkyl chain attached to the benzene ring. It is a colorless to pale yellow liquid with a faint phenolic odor.
Applications DodecylphenolDodecylphenol has been used as an intermediate in the production of various chemicals, including: Nonylphenol ethoxylates (NPEs): These are surfactants used in detergents, cleaners, and other industrial applications. Resins and polymers: Dodecylphenol can be used as a monomer in the production of various resins and polymers. Antioxidants: It can be used as an antioxidant in certain industrial processes.
EDTA (2 Sodium 4 Sodium)
Ethylenediaminetetraacetic acid (EDTA) is a versatile chemical compound with a wide range of applications. It's particularly known for its ability to chelate metal ions, forming stable complexes.
Structure and PropertiesEDTA is a polyamine carboxylic acid that forms strong, water-soluble complexes with a variety of metal ions, including calcium, magnesium, iron, and copper. This chelating ability is due to its multiple nitrogen and oxygen donor atoms that can coordinate with metal ions.
Applications of Ethylenediaminetetraacetic acid
- Water Treatment
- Food Industry
- Pharmaceutical Industry
- Personal Care Products
- Analytical Chemistry
Types of EDTA Salts
EDTA (Dissolvine NA)
EDTA stands for Ethylenediaminetetraacetic acid and is one of the most important chelating agents in various industries, especially in the food, pharmaceutical and cosmetic industries. Its disodium form, Dissolvine NA, is one of the most common forms of this compound.
EDTA is generally a chemical compound with a complex molecular structure that strongly binds to metal ions and forms stable complexes. For this reason, it is known as a chelating agent. Chelating agents are substances that bind free metal ions in solution and prevent them from reacting with other substances.
EDTA (Dissolvine NA) Applications
- Food Industry
- Cosmetic Industry
- Pharmaceutical Industry
- Chemical Industry
Electric Bike Tires
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Vestibulum sagittis orci ac odio dictum tincidunt. Donec ut metus leo. Class aptent taciti sociosqu ad litora torquent per conubia nostra, per inceptos himenaeos. Sed luctus, dui eu sagittis sodales, nulla nibh sagittis augue, vel porttitor diam enim non metus. Vestibulum aliquam augue neque. Phasellus tincidunt odio eget ullamcorper efficitur. Cras placerat ut turpis pellentesque vulputate. Nam sed consequat tortor. Curabitur finibus sapien dolor. Ut eleifend tellus nec erat pulvinar dignissim. Nam non arcu purus. Vivamus et massa massa.
Emulsion
Polyvinyl chloride (PVC) is a widely used thermoplastic polymer made from the polymerization of vinyl chloride monomers (VCM).
PVC is produced by polymerizing vinyl chloride monomers using different techniques.one of these techniques is emulsion polymerization.PVC (Polyvinyl Chloride) emulsion grade is a type of PVC resin produced via the emulsion polymerization process. This method results in very fine particles of PVC, which are ideal for applications requiring a smooth, uniform texture.
StructurePVC emulsion grade is a fine-particle polymer produced through emulsion polymerization, resulting in a high molecular weight material with excellent dispersion and film-forming properties. Its structure consists of small, porous particles that readily absorb plasticizers, making it ideal for flexible and soft applications. The polymer chains in emulsion-grade PVC are densely packed, contributing to its superior adhesion, smooth surface finish, and enhanced mechanical properties. Unlike suspension-grade PVC, which has larger and more irregular particles, emulsion-grade PVC exhibits a uniform texture and lower gelation temperature, making it suitable for applications such as synthetic leather, vinyl flooring, medical gloves, and textile coatings. This structural composition allows for easy processing in plastisols and organosols, ensuring a high degree of flexibility, durability, and aesthetic appeal in the final products.
PropertiesPVC emulsion grade is a fine-particle, high molecular weight polymer known for its excellent dispersion and film-forming properties. It has a small particle size, typically in the range of 0.1–2.0 microns, which allows for superior surface finish and enhanced mechanical strength in end applications. This grade of PVC exhibits good plasticizer absorption, making it ideal for flexible and soft products such as synthetic leather, flooring, coatings, and dip-molded goods. It also offers high viscosity in plastisol form, ensuring uniform application in coatings and pastes. Additionally, PVC emulsion grade demonstrates good chemical resistance, durability, and thermal stability, making it suitable for a wide range of industrial and consumer applications.
Applications of PVC Emulsion Grade:
- Synthetic Leather – Used in the production of artificial leather for furniture, automotive upholstery, and fashion accessories.
- Coatings & Paints – Provides a smooth and durable finish in coatings for fabrics, wallpapers, and flooring.
- Dipping Products – Used in medical gloves, toys, and tool grips due to its excellent film-forming properties.
- Flooring & Wall Coverings – Applied in vinyl flooring, wall coverings, and laminates for enhanced durability and aesthetics.
- Printing Inks – Improves adhesion and flexibility in specialized printing inks.
- Automotive & Construction – Utilized in automotive interiors and flexible membranes in construction applications.
Advantages of PVC Emulsion Grade:✔ Excellent Film Formation – Ensures smooth, uniform coatings and films. ✔ High Plasticizer Absorption – Enables flexibility and softness in final products. ✔ Good Chemical & Weather Resistance – Resistant to moisture, chemicals, and UV exposure, enhancing durability. ✔ Fine Particle Size – Allows superior surface finish and controlled viscosity in plastisol applications. ✔ Versatility – Suitable for a wide range of industrial and consumer applications.
Disadvantages of PVC Emulsion Grade:✖ Environmental Concerns – Contains plasticizers and additives that may cause pollution or health risks if not properly managed. ✖ Processing Sensitivity – Requires precise temperature control during processing to prevent degradation. ✖ Lower Heat Resistance – Can soften or degrade at high temperatures, limiting its use in extreme conditions. ✖ Limited Biodegradability – Like other PVC types, it does not decompose easily, posing disposal challenges.
Engineering ThermoPlastic Vulcanizates (ETPV)
Engineering Thermoplastic Vulcanizates (ETPV) are a class of advanced thermoplastic elastomers (TPEs) that combine the properties of thermoplastics with the resilience of vulcanized rubber. They are formed by dynamically crosslinking an elastomer phase (such as EPDM or NBR) within a thermoplastic matrix (such as polyamide, PBT, or other engineering plastics).
StructureThe structure of Engineering Thermoplastic Vulcanizates (ETPV) consists of a finely dispersed, dynamically crosslinked elastomer phase embedded within a continuous thermoplastic matrix. The elastomer phase, typically made of materials such as EPDM (ethylene propylene diene monomer) or NBR (nitrile butadiene rubber), undergoes vulcanization during melt processing, forming a stable rubber network. This crosslinked rubber phase provides ETPVs with high elasticity, resilience, and excellent mechanical properties. The thermoplastic matrix, often composed of engineering polymers like polyamide (PA), polybutylene terephthalate (PBT), or polyphenylene sulfide (PPS), serves as the continuous phase, giving the material its thermoplastic processability and structural integrity. The intimate interaction between the rubber and thermoplastic phases results in a material that exhibits both the flexibility of elastomers and the durability of engineering plastics. This unique microstructure allows ETPVs to retain their shape after deformation while also being reprocessable and recyclable like conventional thermoplastics.
PropertiesEngineering Thermoplastic Vulcanizates (ETPV) exhibit a unique combination of properties that make them highly versatile for demanding applications. They possess excellent elasticity and flexibility due to their dynamically crosslinked elastomer phase, while the thermoplastic matrix provides high mechanical strength, dimensional stability, and ease of processing. ETPVs offer superior resistance to heat, chemicals, and oils compared to conventional thermoplastic elastomers, making them suitable for high-performance environments such as automotive and industrial applications. They also demonstrate excellent wear and fatigue resistance, ensuring long-term durability under dynamic loading conditions. Unlike traditional rubber materials, ETPVs can be processed using standard thermoplastic techniques like injection molding and extrusion, which enhances manufacturing efficiency. Additionally, they maintain their mechanical integrity across a wide temperature range, making them ideal for applications requiring both flexibility and structural robustness. Their recyclable nature further adds to their sustainability, making them a preferred choice for industries seeking high-performance, cost-effective, and eco-friendly material solutions.
Applications OF ETPV
- Automotive Industry: Seals, gaskets, hoses, under-the-hood components, and weatherstrips.
- Electrical & Electronics: Wire insulation, connectors, and high-performance enclosures.
- Industrial Machinery: Flexible couplings, conveyor belts, vibration dampeners, and seals.
- Medical Devices: Tubing, grips, and sterilizable components.
- Consumer Goods: Sports equipment, handles, and soft-touch applications.
Advantages of ETPVHigh-temperature resistance – Withstands elevated temperatures better than conventional TPVs. Excellent chemical and oil resistance – Suitable for harsh environments. Superior mechanical properties – High strength, durability, and wear resistance. Elasticity and flexibility – Offers rubber-like performance with thermoplastic processability. Easy processing – Can be injection molded, extruded, or thermoformed like standard thermoplastics. Lightweight and recyclable – Environmentally friendly and sustainable alternative to vulcanized rubber.
Disadvantages of ETPVHigher material cost – More expensive compared to standard TPVs and traditional rubbers. Lower flexibility than fully vulcanized rubber – May not be suitable for extreme elasticity needs. Limited performance in extremely high-stress environments – May not replace high-end elastomers in all applications.
Envelop Curing Press
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Vestibulum sagittis orci ac odio dictum tincidunt. Donec ut metus leo. Class aptent taciti sociosqu ad litora torquent per conubia nostra, per inceptos himenaeos. Sed luctus, dui eu sagittis sodales, nulla nibh sagittis augue, vel porttitor diam enim non metus. Vestibulum aliquam augue neque. Phasellus tincidunt odio eget ullamcorper efficitur. Cras placerat ut turpis pellentesque vulputate. Nam sed consequat tortor. Curabitur finibus sapien dolor. Ut eleifend tellus nec erat pulvinar dignissim. Nam non arcu purus. Vivamus et massa massa.